Abstract
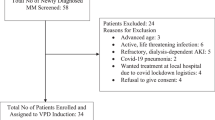
We prospectively evaluated the effect of bortezomib, thalidomide and dexamethasone (VTD) consolidation on bone metabolism of 42 myeloma patients who underwent an autologous stem cell transplantation (ASCT). VTD started on day 100 post ASCT; patients received four cycles of VTD (first block), were followed without treatment for 100 days and then received another four VTD cycles (second block). During this 12-month period, bisphosphonates were not administered. Best response included stringent complete remission (sCR) in 15 (35.7%) patients, complete response (CR) in 13 (30.9%), vgPR in 7 (16.6%), PR in 4 (9.5%), while 3 (7.1%) patients developed a progressive disease (PD). Importantly, 33.3% and 47.6% of patients improved their status of response after the first and second VTD block, respectively. VTD consolidation resulted in a significant reduction of circulating C-terminal cross-linking telopeptide of collagen type I (CTX), soluble receptor activator of the nuclear factor-kappa B ligand (sRANKL) and osteocalcin (OC), whereas bone-specific alkaline phosphatase (bALP) remained stable compared with pre-VTD values. During the study period, only one patient with a PD developed a skeletal-related event (that is, radiation to bone). The median time to progression (TTP) after ASCT was 34 months and the median time of next treatment was 40 months. We conclude that VTD consolidation post ASCT reduces bone resorption and is associated with a very low incidence of skeletal-related events (SREs) despite the absence of bisphosphonates; the later do not appear to be necessary in this context.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
van Rhee F, Szymonifka J, Anaissie E, Nair B, Waheed S, Alsayed Y et al. Total therapy 3 for multiple myeloma: prognostic implications of cumulative dosing and premature discontinuation of VTD maintenance components, bortezomib, thalidomide and dexamethasone, relevant to all phases of therapy. Blood 2010; 116: 1220–1227.
Leleu X, Fouquet G, Hebraud B, Roussel M, Caillot D, Chrétien ML et al. Consolidation with VTd significantly improves the complete remission rate and time to progression following VTdinduction and single autologous stem cell transplantation in multiple myeloma. Leukemia 2013; 27: 2242–2244.
Kim HJ, Yoon SS, Lee DS, Sohn SK, Eom HS, Lee JL et al. Sequential vincristine, adriamycin, dexamethasone (VAD) followed by bortezomib, thalidomide, dexamethasone (VTD) as induction, followed by high-dose therapy with autologous stem cell transplant and consolidation therapy with bortezomib for newly diagnosed multiple myeloma: results of a phase II trial. Ann Hematol 2012; 91: 249–256.
Cavo M, Pantani L, Petrucci MT, Patriarca F, Zamagni E, Donnarumma D et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood 2012; 120: 1597–1600.
Ladetto M, Pagliano G, Ferrero S, Cavallo F, Drandi D, Santo L et al. Major tumor shrinking and persistent molecular remissions after consolidation with bortezomib, thalidomide, and dexamethasone in patients with autografted myeloma. J Clin Oncol 2010; 28: 2077–2084.
Terpos E, Heath DJ, Rahemtulla A, Zervas K, Chantry A, Anagnostopoulos A et al. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br J Haematol 2006; 135: 688–692.
Terpos E, Christoulas D, Kokkoris P, Anargyrou K, Gavriatopoulou M, Migkou M et al. Increased bone mineral density in a subset of patients with relapsed multiple myeloma who received the combination of bortezomib, dexamethasone and zoledronic acid. Ann Oncol 2010; 21: 1561–1562.
Zangari M, Yaccoby S, Pappas L, Cavallo F, Kumar NS, Ranganathan S et al. A prospective evaluation of the biochemical, metabolic, hormonal and structural bone changes associated withbortezomib response in multiple myeloma patients. Haematologica 2011; 96: 333–336.
Terpos E, Morgan G, Dimopoulos M, Drake M, Lentzsch S, Raje N et al. International Myeloma Working Group recommendations for the treatment of multiple myeloma related bone disease. J Clin Oncol 2013; 31: 2347–2357.
Terpos E, Kastritis E, Roussou M, Heath D, Christoulas D, Anagnostopoulos N et al. The combination of bortezomib, melphalan, dexamethasone and intermittent thalidomide is an effective regimen for relapsed/refractory myeloma and is associated with improvement of abnormal bone metabolism and angiogenesis. Leukemia 2008; 22: 2247–2256.
Mhaskar R, Redzepovic J, Wheatley K, Clark OA, Miladinovic B, Glasmacher A et al. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev 2012; 5: CD003188.
Clark RE, Flory AJ, Ion EM, Woodcock BE, Durham BH, Fraser WD . Biochemical markers of bone turnover following high-dose chemotherapy and autografting in multiple myeloma. Blood 2000; 96: 2697–2702.
Terpos E, Politou M, Szydlo R, Nadal E, Avery S, Olavarria E et al. Autologous stem cell transplantation normalizes abnormal bone remodeling and sRANKL/osteoprotegerin ratio in patients with multiple myeloma. Leukemia 2004; 18: 1420–1426.
Politou MC, Heath DJ, Rahemtulla A, Szydlo R, Anagnostopoulos A, Dimopoulos MA et al. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer 2006; 119: 1728–1731.
Terpos E, Mihou D, Szydlo R, Tsimirika K, Karkantaris C, Politou M et al. The combination of intermediate doses of thalidomide with dexamethasone is an effective treatment for patients with refractory/relapsed multiple myeloma and normalizes abnormal bone remodeling, through the reduction of sRANKL/osteoprotegerin ratio. Leukemia 2005; 19: 1969–1976.
Tosi P, Zamagni E, Cellini C, Parente R, Cangini D, Tacchetti P et al. First-line therapy with thalidomide, dexamethasone and zoledronic acid decreases bone resorption markers in patients with multiple myeloma. Eur J Haematol 2006; 76: 399–404.
Gavriatopoulou M, Dimopoulos MA, Christoulas D, Migkou M, Iakovaki M, Gkotzamanidou M et al. Dickkopf-1: a suitable target for the management of myeloma bone disease. Expert Opin Ther Targets 2009; 13: 839–848.
Delforge M, Terpos E, Richardson PG, Shpilberg O, Khuageva NK, Schlag R et al. Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trial in multiple myeloma. Eur J Haematol 2011; 86: 372–384.
Chanan-Khan AA, Giralt S . Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol 2010; 28: 2612–2624.
Harousseau JL, Attal M, Avet-Loiseau H . The role of complete response in multiple myeloma. Blood 2009; 114: 3139–3146.
McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG et al. Lenalidomide after stem cell transplantation for multiple myeloma. N Engl J Med 2012; 366: 1770–1781.
Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T et al. Lenalidomide maintenance after stem cell transplantation for multiple myeloma. N Engl J Med 2012; 366: 1782–1791.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Terpos, E., Christoulas, D., Kastritis, E. et al. VTD consolidation, without bisphosphonates, reduces bone resorption and is associated with a very low incidence of skeletal-related events in myeloma patients post ASCT. Leukemia 28, 928–934 (2014). https://doi.org/10.1038/leu.2013.267
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.267
Keywords
This article is cited by
-
The Proteasome and Myeloma-Associated Bone Disease
Calcified Tissue International (2018)
-
Promotion effect of extracts from plastrum testudinis on alendronate against glucocorticoid-induced osteoporosis in rat spine
Scientific Reports (2017)
-
Myeloma and Bone Disease
Current Osteoporosis Reports (2017)
-
Consolidation and maintenance therapy for multiple myeloma after autologous transplantation: where do we stand?
Bone Marrow Transplantation (2015)
-
Identification of proteins found to be significantly altered when comparing the serum proteome from Multiple Myeloma patients with varying degrees of bone disease
BMC Genomics (2014)