Abstract
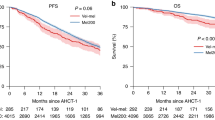
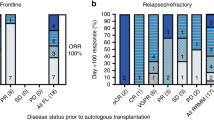
Rapidly progressing relapsed/refractory multiple myeloma (RRMM) patients with compromised marrow have limited treatment options. Thus, non-myeloablative chemotherapy with a stem cell boost (SCB) may provide disease control and hematopoietic improvement as bridge to subsequent therapies. We identified 96 patients who received a SCB between January 2011 and December 2019 at the Mount Sinai Hospital. Patients had a median age of 64 years, received a median of 7 prior lines of therapy and 68 and 42% were triple-class and penta-drug refractory, respectively. Chemotherapy included melphalan (MEL) (n = 16), melphalan + carmustine (BCNU/MEL) (n = 52) or a variant of DCEP (dexamethasone, cyclophosphamide, etoposide, cisplatin) (n = 28). Median time to neutrophil recovery was 10 days and was significantly lower with DCEP (8 days) compared to MEL and BCNU/MEL (10–11 days) (p = 0.0047). Time to progression, progression-free survival and overall survival were 3.19, 2.7 and 8.38 months, respectively. The BCNU/MEL group had the highest response rate of 85% (p = 0.05), clinical benefit rate of 94% (p = 0.0014), progression-free survival of 3.3 months (p = 0.4) and overall survival of 8.7 months (p = 0.5). Sixty-six patients (69%) were bridged to new lines of therapy, including clinical trials. Non-myeloablative chemotherapy with SCB provides rapid disease control and marrow recovery with potential to receive further therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and analysed during the current study are available in the FigShare repository, [https://doi.org/10.6084/m9.figshare.21286503].
References
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. The. N. Engl J Med. 2017;376:1311–20.
Goldschmidt H, Baertsch MA, Schlenzka J, Becker N, Habermehl C, Hielscher T, et al. Salvage autologous transplant and lenalidomide maintenance vs. lenalidomide/dexamethasone for relapsed multiple myeloma: the randomized GMMG phase III trial ReLApsE. Leukemia 2021;35:1134–44.
Cook G, Williams C, Brown JM, Cairns DA, Cavenagh J, Snowden JA, et al. High-dose chemotherapy plus autologous stem-cell transplantation as consolidation therapy in patients with relapsed multiple myeloma after previous autologous stem-cell transplantation (NCRI Myeloma X Relapse [Intensive trial]): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:874–85.
Garderet L, Iacobelli S, Koster L, Goldschmidt H, Johansson JE, Bourhis JH, et al. Outcome of a Salvage Third Autologous Stem Cell Transplantation in Multiple Myeloma. Biol Blood Marrow Transpl. 2018;24:1372–8.
Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, Comenzo RL, et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100). Leukemia 2009;23:1904–12.
Giebel S, Sobczyk-Kruszelnicka M, Blamek S, Sadus-Wojciechowska M, Najda J, Czerw T, et al. Tandem autologous hematopoietic cell transplantation with sequential use of total marrow irradiation and high-dose melphalan in multiple myeloma. Bone Marrow Transpl. 2021;56:1297–304.
Costa LJ, Iacobelli S, Pasquini MC, Modi R, Giaccone L, Blade J, et al. Long-term survival of 1338 MM patients treated with tandem autologous vs. autologous-allogeneic transplantation. Bone Marrow Transpl. 2020;55:1810–6.
Blocka J, Hielscher T, Goldschmidt H, Hillengass J. Response Improvement Rather than Response Status after First Autologous Stem Cell Transplantation Is a Significant Prognostic Factor for Survival Benefit from Tandem Compared with Single Transplantation in Multiple Myeloma Patients. Biol Blood Marrow Transpl. 2020;26:1280–7.
Gagelmann N, Eikema DJ, Koster L, Caillot D, Pioltelli P, Lleonart JB, et al. Tandem Autologous Stem Cell Transplantation Improves Outcomes in Newly Diagnosed Multiple Myeloma with Extramedullary Disease and High-Risk Cytogenetics: A Study from the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2019;25:2134–42.
Jungova A, Vokurka S, Schutzova M, Steinerova K, Mohammadova L, Karas M, et al. Comparison of autologous hematopoietic cell transplantation performed in tandem and in disease relapse in multiple myeloma patients. Neoplasma 2018;65:952–7.
Maffini E, Storer BE, Sandmaier BM, Bruno B, Sahebi F, Shizuru JA, et al. Long-term follow up of tandem autologous-allogeneic hematopoietic cell transplantation for multiple myeloma. Haematologica 2019;104:380–91.
Pick M, Vainstein V, Goldschmidt N, Lavie D, Libster D, Gural A, et al. Daratumumab resistance is frequent in advanced-stage multiple myeloma patients irrespective of CD38 expression and is related to dismal prognosis. Eur J Haematol. 2018;100:494–501.
Gandhi UH, Cornell RF, Lakshman A, Gahvari ZJ, McGehee E, Jagosky MH, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019;33:2266–75.
Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21:207–21.
Chari A, Vogl DT, Gavriatopoulou M, Nooka AK, Yee AJ, Huff CA, et al. Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma. The. N. Engl J Med. 2019;381:727–38.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl. 2009;15:1628–33.
Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 2011;117:4691–5.
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e46.
National Cancer Institute Common Terminology Criteria for Adverse Events [Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood 2016;127:2955–62.
Hagen PA, Stiff P. The Role of Salvage Second Autologous Hematopoietic Cell Transplantation in Relapsed Multiple Myeloma. Biol Blood Marrow Transpl. 2019;25:e98–e107.
Cook G, Ashcroft AJ, Cairns DA, Williams CD, Brown JM, Cavenagh JD, et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open-label, phase 3 trial. Lancet Haematol. 2016;3:e340–51.
Lemieux C, Muffly LS, Iberri DJ, Craig JK, Johnston LJ, Lowsky R, et al. Outcomes after delayed and second autologous stem cell transplant in patients with relapsed multiple myeloma. Bone Marrow Transpl. 2021;56:2664–71.
Garderet L, Morris C, Beksac M, Gahrton G, Schonland S, Yakoub-Agha I, et al. Are Autologous Stem Cell Transplants Still Required to Treat Myeloma in the Era of Novel Therapies? A Review from the Chronic Malignancies Working Party of the EBMT. Biol Blood Marrow Transpl. 2020;26:1559–66.
Kazandjian D, Landgren O. Delaying the use of high-dose melphalan with stem cell rescue in multiple myeloma is ready for prime time. Clin Adv Hematol Oncol. 2019;17:559–68.
Martino M, Tripepi G, Messina G, Vincelli ID, Console G, Recchia AG, et al. A phase II, single-arm, prospective study of bendamustine plus melphalan conditioning for second autologous stem cell transplantation in de novo multiple myeloma patients through a tandem transplant strategy. Bone Marrow Transpl. 2016;51:1197–203.
Roussel M, Lauwers-Cances V, Macro M, Leleu X, Royer B, Hulin C, et al. Bortezomib and high-dose melphalan conditioning regimen in frontline multiple myeloma: an IFM randomized phase 3 study. Blood 2022;139:2747–57.
Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood 2020;136:936–45.
Musso M, Messina G, Marcacci G, Crescimanno A, Console G, Donnarumma D, et al. High-Dose Melphalan Plus Thiotepa as Conditioning Regimen before Second Autologous Stem Cell Transplantation for “De Novo” Multiple Myeloma Patients: A Phase II Study. Biol Blood Marrow Transpl. 2015;21:1932–8.
Lahuerta JJ, Mateos MV, Martinez-Lopez J, Grande C, de la Rubia J, Rosinol L, et al. Busulfan 12 mg/kg plus melphalan 140 mg/m2 versus melphalan 200 mg/m2 as conditioning regimens for autologous transplantation in newly diagnosed multiple myeloma patients included in the PETHEMA/GEM2000 study. Haematologica 2010;95:1913–20.
Gerrie AS, Mikhael JR, Cheng L, Jiang H, Kukreti V, Panzarella T, et al. D(T)PACE as salvage therapy for aggressive or refractory multiple myeloma. Br J Haematol. 2013;161:802–10.
Abdallah AO, Sigle M, Mohyuddin GR, Coggins E, Remker C, Shune L, et al. Outcomes of VD-PACE With Immunomodulatory Agent as a Salvage Therapy for Relapsed/Refractory Multiple Myeloma. Clin lymphoma, myeloma Leuk. 2021;21:e220–e6.
Griffin PT, Ho VQ, Fulp W, Nishihori T, Shain KH, Alsina M, et al. A comparison of salvage infusional chemotherapy regimens for recurrent/refractory multiple myeloma. Cancer 2015;121:3622–30.
Goldsmith SR, Fiala MA, Wang B, Schroeder MA, Wildes TM, Ghobadi A, et al. DCEP and bendamustine/prednisone as salvage therapy for quad- and penta-refractory multiple myeloma. Ann Hematol. 2020;99:1041–8.
Park S, Lee SJ, Jung CW, Jang JH, Kim SJ, Kim WS, et al. DCEP for relapsed or refractory multiple myeloma after therapy with novel agents. Ann Hematol. 2014;93:99–105.
Yuen HLA, Low MSY, Fedele P, Kalff A, Walker P, Bergin K, et al. DCEP as a bridge to ongoing therapies for advanced relapsed and/or refractory multiple myeloma. Leuk lymphoma. 2018;59:2842–6.
Lakshman A, Singh PP, Rajkumar SV, Dispenzieri A, Lacy MQ, Gertz MA, et al. Efficacy of VDT PACE-like regimens in treatment of relapsed/refractory multiple myeloma. Am J Hematol. 2018;93:179–86.
Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 2021;398:314–24.
Munshi NC, Anderson LD Jr., Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl J Med. 2021;384:705–16.
Author information
Authors and Affiliations
Contributions
Conceptualization: THM and JR; Methodology: EM; Investigation: THM, ST, BP, MR, RJ and AS; Writing – Original Draft: THM; Writing – Review & Editing: all authors; Resources: JR; Supervision: JR.
Corresponding author
Ethics declarations
Competing interests
THM received advisory board fees from Legend Biotech. LS received consulting fees from Takeda. SR received honoraria from Karyopharm and Janssen, and advisory board fees from Karyopharm and Celgene/Bristol Myers Squibb, and research support from Janssen, Celgene/Bristol Myers Squibb, C4 Therapeutics. AR received advisory board fees from Celgene/Bristol Myers Squibb, Janssen, Sanofi, and GlaxoSmithKline. HJC is an employee of the Multiple Myeloma Research Foundation, and received research funding from Celgene/Bristol Myers Squibb, and Takeda. CR received consulting fees from Janssen, Artica, Takeda, Amgen, Karyopham, and Caelum Biosciences. SP received advisory board fees from GRAIL and research support from Celgene/Bristol Myers Squibb, Amgen, and Karyopharm. A.C. received consulting fees from Amgen, Celgene/Bristol Myers Squibb, Janssen, Karyopharm, and Takeda, and advisory board fees from Amgen, Celgene/Bristol Myers Squibb, Janssen, Karyopharm, Takeda, Sanofi, and Seattle Genetics, and research support from Amgen, Array Biopharma, Celgene/Bristol Myers Squibb, GlaxoSmithKline, Janssen, Takeda, Novartis, Oncoceutics, Pharmacyclics and Seattle Genetics. SJ received advisory board fees and consulting fees from Celgene/Bristol Myers Squibb, Janssen, Legend Biotech, Karyopharm, Sanofi and Takeda. J.R. received speaking fees from Celgene/Bristol Myers Squibb, Sanofi, and Janssen, and advisory board fees from Celgene/Bristol Myers Squibb, Janssen, Celgene/Bristol Myers Squibb, Karyopharm, Sanofi, X4 Pharmaceuticals, Oncopeptides, Adaptive Biotechnologies, Secura Bio, Astrazeneca, and Takeda, and consulting fees from Celgene/Bristol Myers Squibb, Secura Bio, and Oncopeptides. EM, ST, BP, MR, RJ, and AS declare no potential conflict of interest.
Ethical approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mouhieddine, T.H., Moshier, E., Thibaud, S. et al. Bridging advanced myeloma patients to subsequent treatments and clinical trials with classical chemotherapy and stem cell support. Bone Marrow Transplant 58, 80–86 (2023). https://doi.org/10.1038/s41409-022-01848-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01848-7