Abstract
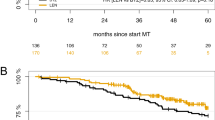
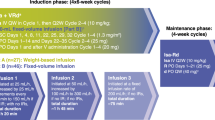
Bortezomib, lenalidomide, and dexamethasone (VRD) induction is standard prior to autologous hematopoietic cell transplantation (auto-HCT) in newly diagnosed, high-risk multiple myeloma (ND-HRMM). Carfilzomib (K) is another proteasome inhibitor approved for MM. In this single-center, retrospective analysis, we compared outcomes in ND-HRMM with pre-transplant KRD or VRD induction. High-risk was defined by t(4:14), t(14:16), 1q21 gain/amplification, or del(17p). Primary endpoints were progression-free (PFS) and overall survival (OS). Of 121 ND-HRMM patients, 63 received KRD, and 58 received VRD. Post-induction, complete (CR), very good partial (VGPR), partial response (PR), and overall response (ORR) rates were 23.8%/49.2%/25.4%/98.4% with KRD, and 19%/46.6%/27.6%/93.1% with VRD. At day 100 post-auto-HCT, these were 38.1%/42.9%/19%/100% with KRD, versus 35.1%/49.1%/12.3%/94.8% with VRD. Pre-auto-HCT, 11 (18.3%) KRD and 7 (12.5%) VRD patients had minimal residual disease (MRD)-negative CR (p = 0.45). Post-auto-HCT, 14 (41.2%) and 13 (43.3%) patients had MRD-negative CR (p = 1.000). Median PFS was 38.2 (95%CI 28.7-NA) and 45.9 months (95%CI 43.2-NA) for KRD and VRD, respectively (p = 0.25). Respective 3-year PFS and OS were 53.5% (95%CI 41.1–69.6) and 95.2% (95%CI 90–100) for KRD and 64% (95%CI 51.6–79.5) and 84.2% (95%CI 73.5–96.3, p = 0.30) for VRD. Overall, KRD induction pre-auto-HCT does not improve outcomes. Prospective, randomized studies are needed to confirm these findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All the underlying data presented in this study is available upon reasonable request to the corresponding author by email.
References
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N. Engl J Med. 2017;376:1311–20.
Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–86.
Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012;119:4375–82.
Roussel M, Lauwers-Cances V, Robillard N, Hulin C, Leleu X, Benboubker L, et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the Intergroupe Francophone du Myelome. J Clin Oncol. 2014;32:2712–7.
Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet 2017;389:519–27.
Rosiñol L, Oriol A, Rios R, Sureda A, Blanchard MJ, Hernández MT, et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood 2019;134:1337–45.
Joseph NS, Kaufman JL, Dhodapkar MV, Hofmeister CC, Almaula DK, Heffner LT, et al. Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J Clin Oncol. 2020;38:1928–37.
Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–90.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N. Engl J Med. 2014;372:142–52.
Jasielec JK, Kubicki T, Raje N, Vij R, Reece D, Berdeja J, et al. Carfilzomib, lenalidomide, and dexamethasone plus transplant in newly diagnosed multiple myeloma. Blood 2020;136:2513–23.
Kumar SK, Jacobus SJ, Cohen AD, Weiss M, Callander N, Singh AK, et al. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21:1317–30.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46.
Garderet L, D’Souza A, Jacobs P, van Biezen A, Schönland S, Kroeger N, et al. Response assessment in myeloma: practical manual on consistent reporting in an era of dramatic therapeutic advances. Biol Blood Marrow Transplant. 2017;23:1193–202.
Gay F, Mina R, Rota-Scalabrini D, Galli M, Belotti A, Zamagni E, et al. Carfilzomib-based induction/consolidation with or without autologous transplant (ASCT) followed by lenalidomide (R) or carfilzomib-lenalidomide (KR) maintenance: efficacy in high-risk patients. J Clin Oncol. 2021;39:8002.
Saini N, Bashir Q, Milton DR, Tang G, Delgado R, Rondon G, et al. Busulfan and melphalan conditioning is superior to melphalan alone in autologous stem cell transplantation for high-risk MM. Blood Adv. 2020;4:4834–7.
Bashir Q, Thall PF, Milton DR, Fox PS, Kawedia JD, Kebriaei P, et al. Conditioning with busulfan plus melphalan versus melphalan alone before autologous haemopoietic cell transplantation for multiple myeloma: an open-label, randomised, phase 3 trial. Lancet Haematol. 2019;6:e266–75.
Usmani SZ, Hoering A, Ailawadhi S, Sexton R, Lipe B, Hita SF, et al. Bortezomib, lenalidomide, and dexamethasone with or without elotuzumab in patients with untreated, high-risk multiple myeloma (SWOG-1211): primary analysis of a randomised, phase 2 trial. Lancet Haematol. 2021;8:e45–54.
Leypoldt LB, Besemer B, Asemissen AM, Hänel M, Blau IW, Görner M, et al. Isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in front-line treatment of high-risk multiple myeloma: interim analysis of the GMMG-CONCEPT trial. Leukemia 2022;36:885–8.
Acknowledgements
EEM and RZO would like to acknowledge support from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, and from the Riney Family Multiple Myeloma Research Program.
Author information
Authors and Affiliations
Contributions
MRG and MHQ designed the study, interpreted the data, and wrote the manuscript. JM and RB completed the statistical analysis, interpreted the data, and edited the manuscript. MR, MRT, QB, SAS, NS, JR, YN, RM, KR, GT, PL, HCL, KKP, MRU, GPK, EEM, PK, SKT, DMW, EJS, REC, and RZO provided critical review of the study and edited the manuscript. All authors have approved of the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and patient consent statement
This retrospective chart review was completed following approval by the MD Anderson Institutional Review Board and in accordance with the Declaration of Helsinki and the 1996 Health Insurance Portability and Accountability Act guidelines.
Competing interests
MHQ has research support from Janssen, Angiocrine, NexImmune, and Amgen, and consulting or advisory board with Bioclinica, Bristol–Myers Squib, and Oncopeptides. QB has research support from Stemline, Acrotech, and Takeda, and consulting or advisory board with Takeda, KITE, Amgen, Purdue, and Stemline. YN has research support from Astra Zeneca, Affimed, Novartis, and Secura Bio, and consulting with Affimed. KR has research support from Pharmacyclics and Affimed, personal fees and/or advisory board with Gemoab, AvengeBio, Virogin, GSK, Navan Technologies, and Bayer, and a patent license, royalties, and research agreement from Takeda for work outside of this study. HCL has research support and/or consulting with Daiichi Sankyo, Regeneron, Amgen, Celgene, Janssen, Takeda, GSK, Genentech, Sanofi, BMS, Immunitas Legend Biotech, Oncopeptides, and Karyopharm. KKP has research support and/or consulting from Abbvie, Cellectis, Poseida Therapeutics, Bristol–Myers Squibb, Arcellx, Precision Biosciences, Nektar, Takeda, Celgene, Janssen, and Oncopeptides. GPK has research support from BMS and Janssen, and consulting or advisory board with Karyopharm. EEM has research support from Merck, JW Pharma, Novartis, Quest Diagnostics, and Sanofi, and consulting with Adaptive Biotechnologies, GSK, Sanofi, BMS, and Takeda. PK has research support from Ziopharm and Amgen, and consulting or advisory board with Jazz, Pfizer, Novartis, and KITE. SKT has research support from Genentech, Xencor, Acerta, X4 Pharma, Ascentage Pharma and BMS, and advisory board/board of directors with Beigen, Pharmacyclics, and X4 Pharma. RZO is a founder of Asylia Therapeutics, research support from BioTheryX, CARsgen Therapeutics, Exelixis, Celgene, Janssen, Sanofi, and Takeda, and consulting/advisory board with STATinMED, Sanofi, Servier, Takeda, Amgen, Astra Zeneca, BMS, Celgene, EcoR1 Capital, Genzyme, Forma Therapeutics, GSK, Ionis, Janssen, Juno Therapeutics, KITE, Legend Biotech, Regeneron, and Molecular Partners. EJS is co-inventor for a patent licensed to Takeda and advisory board/consulting with Adaptimmune, Axio, Navan, Novartis, and Magenta. The other authors have not declared a competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gaballa, M.R., Ma, J., Rauf, M. et al. KRD vs. VRD as induction before autologous hematopoietic progenitor cell transplantation for high-risk multiple myeloma. Bone Marrow Transplant 57, 1142–1149 (2022). https://doi.org/10.1038/s41409-022-01697-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01697-4