Abstract
Objective:
The efficacy of inhaled steroids in spontaneously breathing infants with established bronchopulmonary dysplasia (BPD) is debatable. The inhaled steroid hydrofluoalkane-beclomethasone dipropionate (QVAR) is unique in its small particle size that results in higher lung deposition. Our objective was to determine if inhaled QVAR could decrease respiratory rehospitalizations of infants with established BPD.
Study Design:
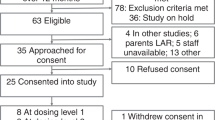
Double-blind, randomized placebo-controlled, multicenter pilot study. Preterm infants with moderate-to-severe BPD were randomized to inhaled QVAR 100 μg per dose or placebo twice daily via Aerochamber with face mask. Treatment was administered daily from recruitment at 36 weeks post menstrual age until 3 months post discharge. Analysis was carried out by intention to treat.
Results:
The QVAR (n=18) and placebo (n=20) groups were comparable in birth and recruitment characteristics. Length of stay (108.5±26.3 vs 108.7±36.0 days) and infants requiring oxygen at discharge (5/17 vs 6/19) or at study end (0/17 vs 2/19) were comparable. Respiratory rehospitalizations/infant (0.1±0.5 vs 0.4±0.6), rehospitalization days (0.5±1.5 vs 4.1±10.3), and post-discharge additive inhaled (0.3±0.9 vs 6.4±21.5 days), systemic (0.7±2.8 vs 1.0±1.4 days) and combined (inhaled/systemic) steroids (1.0±2.9 vs 7.8±25.8 days) tended to be lower in the QVAR compared with the placebo group. Blood pressure, height and weight gain, and urine cortisol/creatinine ratio at study end were comparable between groups.
Conclusions:
Our study was unable to detect a significant effect of inhaled QVAR on the respiratory course of established BPD. The study was underpowered. Possible benefits of QVAR could be masked by a tendency toward higher use of additional steroids in the placebo group.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Slaughter JL, Stenger MR, Reagan PB, Jadcherla SR . Utilization of inhaled corticosteroids for infants with bronchopulmonary dysplasia. PLoS One 2014; 9 (9): e106838.
Allen J, Zwerdling R, Ehrenkranz R, Gaultier C, Geggel R, Greenough A et al. Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med 2003; 168 (3): 356–396.
Lister P, Iles R, Shaw B, Ducharme F . Inhaled steroids for neonatal chronic lung disease. Cochrane Database Syst Rev 2000; (3): CD002311.
Onland W, Offringa M, van Kaam A . Late (≥ 7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev 2012; (4): CD002311.
Yuksel B, Greenough A . Randomised trial of inhaled steroids in preterm infants with respiratory symptoms at follow up. Thorax 1992; 47 (11): 910–913.
Beresford MW, Primhak R, Subheddar NV, Shaw NJ . Randomised double blind placebo controlled trial of inhaled fluticasone propionate in infants with chronic lung disease. Arch Dis Child Fetal Neonatal Ed 2002; 87 (1): F62–F63.
Baraldi E, Filippone M . Chronic lung disease after premature birth. N Engl J Med 2007; 357 (19): 1946–1955.
Jobe AH, Bancalari E . Bronchopulmonary dysplasia. NICHD-NHLBI-ORD Workshop. Am J Respir Crit Care Med 2001; 163 (7): 1723–1729.
Jobe AH . The new bronchopulmonary dysplasia. Curr Opin Pediatr 2011; 23 (2): 167–172.
Baud O, Maury L, Lebail F, Ramful D, El Moussawi F, Nicaise C et alPREMILOC trial study group. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet 2016; 387 (10030) 1827–1836.
Bassler D, Plavka R, Shinwell ES, Hallman M, Jarreau PH, Carnielli V et alNEUROSIS Trial Group. Early inhaled budesonide for the prevention of bronchopulmonary dysplasia. N Engl J Med 2015; 373 (16): 1497–1506.
Leach CL, Davidson PJ, Hasselquist BE, Boudreau RJ . Lung deposition of hydrofluoroalkane-134a beclomethasone is greater than that of chlorofluorocarbon fluticasone and chlorofluorocarbon beclomethasone: a cross-over study in healthy volunteers. Chest 2002; 122 (2): 510–516.
de Vries TW, Rottier BL, Gjaltema D, Hagedoorn P, Frijlink HW, de Boer AH . Comparative in vitro evaluation of four corticosteroid metered dose inhalers: consistency of delivered dose and particle size distribution. Respir Med 2009; 103 (8): 1167–1173.
Leach CL, Colice GL . A pilot study to assess lung deposition of HFA-beclomethasone and CFC-beclomethasone from a pressurized metered dose inhaler with and without add-on spacers and using varying breathhold times. J Aerosol Med Pulm Drug Deliv 2010; 23 (6): 355–6115.
Thorsson L, Edsbäcker S, Conradson TB . Lung deposition of budesonide from Turbuhaler is twice that from a pressurized metered-dose inhaler P-MDI. Eur Respir J 1994; 7 (10): 1839–1844.
Janssens HM, De Jongste JC, Hop WC, Tiddens HA . Extra-fine particles improve lung delivery of inhaled steroids in infants: a study in an upper airway model. Chest 2003; 123 (6): 2083–2088.
Devadason SG, Huang T, Walker S, Troedson R, Le Souëf PN . Distribution of technetium-99 m-labelled QVAR delivered using an Autohaler device in children. Eur Respir J 2003; 21 (6): 1007–1011.
Vanden Burgt JA, Busse WW, Martin RJ, Szefler SJ, Donnell D . Efficacy and safety overview of a new inhaled corticosteroid, QVAR (hydrofluoroalkane-beclomethasone extrafine inhalation aerosol), in asthma. J Allergy Clin Immunol 2000; 106 (6): 1209–1226.
Cole CH, Mitchell JP, Foley MP, Nagel MW . Hydrofluoroalkane-beclomethasone versus chlorofluocarbon-beclomethasone delivery in neonatal models. Arch Dis Child Fetal Neonatal Ed 2004; 89 (5): F417–F418.
Robroeks CM, van de Kant KD, van Vliet D, Kester AD, Hendriks HJ, Damoiseaux JG et al. Comparison of the anti-inflammatory effects of extra-fine hydrofluoroalkane-beclomethasone vs fluticasone dry powder inhaler on exhaled inflammatory markers in childhood asthma. Ann Allergy Asthma Immunol 2008; 100 (6): 601–607.
Fireman P, Prenner BM, Vincken W, Demedts M, Mol SJ, Cohen RM . Long-term safety and efficacy of a chlorofluorocarbon-free beclomethasone dipropionate extrafine aerosol. Ann Allergy Asthma Immunol 2001; 86 (5): 557–565.
Juniper EF, Price DB, Stampone PA, Creemers JP, Mol SJ, Fireman P . Clinically important improvements in asthma-specific quality of life, but no difference in conventional clinical indexes in patients changed from conventional beclomethasone dipropionate to approximately half the dose of extrafine beclomethasone dipropionate. Chest 2002; 121 (6): 1824–1832.
Price D, Martin RJ, Barnes N, Dorinsky P, Israel E, Roche N et al. Prescribing practices and asthma control with hydrofluoroalkane-beclomethasone and fluticasone: a real-world observational study. J Allergy Clin Immunol 2010; 126 (3): 511–518.
Petersen R, Agertoft L, Pedersen S . Treatment of exercise-induced asthma with beclomethasone dipropionate in children with asthma. Eur Respir J 2004; 24 (6): 932–937.
Worth H, Muir JF, Pieters WR . Comparison of hydrofluoroalkane-beclomethasone dipropionate Autohaler with budesonide Turbuhaler in asthma control. Respiration 2001; 68 (5): 517–526.
Randell TL, Donaghue KC, Ambler GR, Cowell CT, Fitzgerald DA, van Asperen PP . Safety of the newer inhaled corticosteroids in childhood asthma. Paediatr Drugs 2003; 5 (7): 481–504.
Smith VC, Zupancic JA, McCormick MC, Croen LA, Greene J, Escobar GJ et al. Rehospitalizations in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr 2004; 144 (6): 799–803.
Chien YH, Tsao PN, Chou HC, Tang JR, Tsou KI . Rehospitalization of extremely-low-birth-weight infants in first 2 years of life. Early Hum Dev 2002; 66 (1): 33–40.
Dugas MA, Nguyen D, Frenette L, Lachance C, St-Onge O, Fougères A et al. Fluticasone inhalation in moderate cases of bronchopulmonary dysplasia. Pediatrics 2005; 115 (5): e566–e572.
Giep T, Raibble P, Zuerlein T, Schwartz ID . Trial of beclomethasone dipropionate by metered-dose inhaler in ventilator-dependent neonates less than 1500 grams. Am J Perinatol 1996; 13 (1): 5–9.
Global Initiative for Asthma. For Children 5 Years and Younger. A Guide for Healthcare Professionals. Updated 2015. Available from http://www.ginasthma.org.
Acknowledgements
The study was not funded. Teva supplied the QVAR and the placebo, and Trupharm supplied the Aerochambers for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kugelman, A., Peniakov, M., Zangen, S. et al. Inhaled hydrofluoalkane-beclomethasone dipropionate in bronchopulmonary dysplasia. A double-blind, randomized, controlled pilot study. J Perinatol 37, 197–202 (2017). https://doi.org/10.1038/jp.2016.177
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.177