Abstract
Objective:
The objectives of this study were to assess (i) the agreement between servo-control temperature (Tfeedback) and rectal temperature (Tre) and (ii) the distribution of regional skin temperatures (Tsk) of neonates nursed under a radiant warmer (RW) in a neonatal intensive care unit.
Study design:
An observational study of 13 neonates nursed under a RW device set to servo-control mode (Tfeedback set-point: 36.5 °C) who were monitored for Tfeedback, Tre and Tsk at six sites for a period of 105 min.
Results:
Mean bias for Tfeedback relative to Tre was +0.01 °C, but 95% limits of agreement were ±0.99 °C, and only 66% of Tfeedback values were within the acceptable limits determined a priori (±0.5 °C). Tfeedback was maintained within a range of 1.4 °C (35.9 to 37.3 °C), whereas the range observed for regional skin temperatures spanned from 9.5 °C (27.3 to 36.8 °C; foot) to 4.8 °C (33.1 to 37.9 °C; chest).
Conclusion:
Although Tfeedback is maintained within narrow limits, the level of agreement with Tre is poor. In addition, large fluctuations in regional skin temperatures occur with a consistent caudal-to-rostral temperature gradient, irrespective of Tfeedback.
Similar content being viewed by others
Introduction
For newborns, optimizing thermal homeostasis is imperative for their survival and growth.1 Newborns are typically predisposed to excessive heat dissipation because of an immature thermoregulatory system and a large surface area-to-body mass ratio that is up to four times greater at birth than during adulthood.2 Accordingly, newborns have an elevated risk of hypothermia, which is known to adversely affect survival.1 In neonatal intensive care units (NICU), radiant warmers (RW) are often used to provide passive heat exchange via radiant energy as a means of ensuring the maintenance of a stable core body temperature. The primary benefits of using a RW device as opposed to an incubator is that it provides high accessibility to the patient with minimal disturbance to their thermal environment.3
Radiant heat output from a typical RW device is regulated according to a servo-controlled feedback signal from a single local skin temperature measurement taken over the anterior portion of the abdominal wall close to the axilla (Tfeedback). Traditionally, axilla temperature has been considered to be a reliable representation of core temperature of a newborn.2, 4 As such, the measurement of Tfeedback alone as part of a RW system has been considered by some to void the need for any other additional core temperature measurements by NICU nursing staff.2 However, others have demonstrated that even a well-positioned axilla temperature measurement can be an inaccurate indicator of an infant’s true deep core temperature (that is, rectal temperature (Tre)), even under stable environmental conditions.5 As such, the utility of using a single measure of local skin temperature to derive an accurate representation of core6 and even whole-body skin temperatures7, 8 in this population, particularly when nursed under a RW device that consistently changes radiant heat output, seems highly questionable.6 Indeed, a case study of the accidental overheating of a newborn in a NICU under a servo-controlled RW was recently reported by our group, with large elevations in skin temperature and attendant physiological strain observed despite little change in Tfeedback.8
Given that Tfeedback measurements of a RW device are routinely interpreted as an indication of an infant’s core temperature,9 it is necessary to assess the level of agreement between Tfeedback and a deeper core temperature measurement (that is, Tre) as RW output changes. Furthermore, the heterogeneity of local skin temperatures measured on different skin regions of a neonate nursed under a servo-controlled RW has not yet been fully determined.2, 10 Thus, a second aim of the present study was to map regional skin temperatures across neonates nursed under a RW device.
Methods
Patients
Prior to the commencement of this observational study, ethical approval was obtained from the Research Ethics Board of the Children’s Hospital of Eastern Ontario Research Institute. Written and informed consent was obtained from the legal guardians of 13 neonates (10 males, 3 females) admitted to a tertiary-level NICU at the Children’s Hospital of Eastern Ontario, in Ottawa, Canada (Table 1). Reasons for NICU admission were respiratory distress syndrome (8), Ebstein’s anomaly (1), Patent ductus arteriosus (1), Pyloric stenosis (1), Hydrops fetalis/ascites (1) and Necrotizing enterocolitis (1). All of these conditions were representative of typical reasons for NICU admission. Inclusion criteria for this study were: a postnatal age of 0 to 30 days and the patient had to be nursed at the time under a standard infant RW (Giraffe Warmer, GE Healthcare, Helsinki, Finland) set to servo-control mode at a Tfeedback temperature of 36.5 °C, measured on the skin at a single point over the anterior portion of the abdominal wall close to the axilla according to standard care procedures.11 Patients were excluded if they were hemodynamically unstable, on high-frequency ventilation, considered immunocompromised, had any medical conditions that had confounding thermoregulatory effects (for example, hypoxic-ischemic encephalopathy, malignant hyperthermia, neonatal sepsis and so on), had malformations that precluded the use of any temperature probe (for example, gastroschisis, peripheral skin lesions and so on) or if their RW was set on ‘manual’ mode. All patients were mechanically ventilated using a standard infant ventilator (DrägerBabylogVN500, Dräger Medical, Lübeck, Germany) and were sedated via intravenous administration of fentanyl (0.5 to 2 μg kg−1 h−1) on the basis of their pain score, as per standard care procedures.
Instrumentation
The RW servo-control temperature was regulated by local skin temperature measured at a single point over the anterior portion of the abdominal wall (Tfeedback) close to the axilla, in line with the standard care procedures of the NICU and attached by a qualified registered nurse. The infant’s arm was adducted after placement of the Tfeedback probe to avoid any further exposure to changes in environmental and radiant temperature. Skin temperature was measured over the right side of the body, at six standardized anatomical sites (forehead (Tforehead), arm (Tarm), chest (Tchest), abdomen (Tabd), thigh (Tthigh) and foot (Tfoot)) based on the work of Karlsson.12 All skin temperatures and Tfeedback were measured using adhesive thermistor probes (400 series, Model# STS-400, Smiths Medical, Dublin, OH, USA) and were insulated to protect the probes from any direct radiant heat using a reflective covering. Rectal temperature (Tre) was measured using a pediatric general-purpose thermistor probe (400 series, Model# ER400-9, Smiths Medical) inserted to a minimum depth of 3 cm past the anal sphincter, as previously reported.11, 13 Measurements of Tfeedback, Tsk at all sites and Tre were all recorded using a National Instruments data acquisition module (model NI cDAQ-9172) at a sampling rate of 1 Hz. Data were simultaneously displayed and recorded using customized Lab-VIEW software (Version 8.6.1, National Instruments, Austin,TX, USA).
Protocol
Prior to data collection, anthropometric measures (mass, length, age, gestational age and sex) were documented from a standardized case report form for each individual participant. Skin temperature sensors were then placed on the six anatomical sites previously identified on the right side of the body. Then, a registered nurse inserted a rectal thermistor, while also ensuring that the patient remained centrally placed under the RW device. The total duration of data collection was 105 min, during which time the infants were left undisturbed by the researcher. The NICU environmental conditions were similar for all trials (Ta: 23.5±0.4 °C; RH: 34±12%; Pbar: 759±3 mm Hg).
Statistical analysis
A Bland-Altman plot was used to assess the agreement between Tfeedback and Tre by calculating the mean bias and limits of agreement,14 with our reference measure (Tre) on the x axis.15 The difference between Tre and Tfeedback, with 95% probability, will lie within the respective limits of agreement which were calculated for each individual every 5 min by multiplying the standard deviation of the mean difference between Tfeedback and Tre by 1.96. The acceptable limits of agreement were determined a priori as ±0.5 °C.16, 17, 18, 19 The variability and range in Tfeedback, Tre, Tforehead, Tarm, Tchest, Tabd, Tthigh and Tfoot were assessed by deriving the median and maximum/minimum values (range) of the upper and lower quartile values of each participant, and the median values of each participant. All analyses were performed using SPSS version 22 (IBM Corp., Armonk, NY, USA).
Results
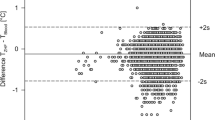
Rectal temperature ranged from 35.16 to 37.50 °C. When comparing Tfeedback values against concurrent Tre values, the mean bias was −0.01 °C. However, this bias was systematically altered by Tre (r=0.95, P<0.001). The 95% limits of agreement for the estimation of Tre using Tfeedback were +0.97 °C to −1.00 °C. Of all Tfeedback values, 66% were within ±0.5 °C of Tre—the acceptable limits of agreement determined a priori. A Bland-Altman plot for Tfeedback relative to Tre is given in Figure 1.
Bland-Altman plot illustrating the mean bias (dotted line) and 95% confidence intervals (±1.96 s.d.: solid lines) for predicted rectal temperature (Tre) using the servo‐control feedback temperature (Tfeedback) of a RW device. Gray band indicates the a priori acceptable limits of agreement (±0.5 °C) for estimating the core temperature.16
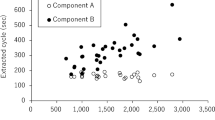
Regional skin temperatures on the forehead, arm, chest, abdomen, thigh and foot, as well as concurrent Tfeedback and Tre values are illustrated in Figure 2. Median values are given for the lower and upper quartile of all participants as well as the median of the median values of all participants. The overall range (that is, the difference between the lowest and highest values measured within a given region) for Tfeedback, which was tightly regulated by the RW device, was low (1.4 °C: 37.3 to 35.9 °C) in comparison with regional skin temperatures, which were between threefold (Tchest; 4.8 °C: 33.1 to 37.9 °C) and sixfold (Tfoot; 9.5 °C: 27.3 to 36.8 °C) greater. In all cases, a caudal-to-rostral temperature gradient was observed with the forehead, chest and abdomen 1 to 4 °C warmer than the thigh and foot. In the most extreme cases, Tfoot was 6.1 °C lower than Tforehead and simultaneously 4.2 °C lower than Tfeedback.
Discussion
Infant RW rely on the feedback from a single local skin temperature measured over the anterior portion of the abdominal wall (Tfeedback) according to standard care procedures to regulate radiant heat output, presumably owing to the notion that this temperature, which is close to the axilla, is indicative of infant thermal status.2, 4, 20 The present study demonstrates how poorly Tfeedback represents deep core temperature (Tre) for neonates nursed under a RW in a NICU (Figure 1).21, 22, 23 Furthermore, large differences in skin temperature between body regions were observed both when Tfeedback was the highest (and the RW was fully on) and the lowest (when the RW was fully off), implying an uneven exposure of radiant heat across the body leading to much cooler peripheral temperatures (feet) relative to skin regions more central to the RW (forehead, chest and torso).
When using Tfeedback as a surrogate measure of Tre, the 95% limits of agreement far exceed those considered acceptable for clinical practice (±0.5 °C).16 Indeed, only 66% of all values were within these acceptable limits as determined a priori (gray band—Figure 1). In addition, measurements of Tfeedback systematically overestimated Tre at Tre values <36.0 °C and similarly underestimate Tre measurements when values exceeded 37.0 °C. Indeed, it is clear from the present data that although Tfeedback remains relatively stable throughout all trials, rectal temperature appears to vary somewhat independently. For example as illustrated in Figure 1, when Tre was 35.5 °C, Tfeedback was >1.0 °C higher and radiant heat output from the RW device would have been mostly downregulated (because the RW Tfeedback was not below its set-point of 36.5 °C) when in fact more warming was needed. On the other hand, when Tre was above 37.0 °C, Tfeedback was in ~50% of cases more than 0.5 °C lower and therefore below the set-point value of 36.5 °C; consequently radiant heat output from the RW device would have remained mostly upregulated when more warming was not actually needed. The reason for these observations are unclear, however, the thermal inertia of the body likely leads to a delay in body shell-to-core heat transfer and thus changes in Tfeedback and Tre that are partially out of phase. Although it may be tempting for caregivers to rely on this single skin temperature for an assessment of whole-body thermal status, our data clearly demonstrate that Tfeedback should be interpreted with extreme caution in the absence of a parallel measure of deep core temperature. Moreover, the utility of Tfeedback for regulating the required radiant heat output is also limited.
Although modulations in RW output ensured only modest variation in Tfeedback, large variations in skin temperatures were observed across the body. Moreover, irrespective of Tfeedback, lower limb skin temperatures were consistently cooler and demonstrated a larger temperature range than those regions more central to the RW (Figure 2), which were mostly likely exposed to a greater flux of radiant heat. The highest skin temperatures recorded were on the abdomen (39.0 °C), forehead (38.9 °C) and upper arm (38.8 °C), and far exceeded the Tfeedback value measured concurrently (37.3 °C). Although these skin temperatures were not sufficiently high to cause skin damage (49 °C 24), the dissociation between Tfeedback and these skin temperatures does present a potential risk to the wellbeing of an infant nursed under a RW device if other regional temperatures are not monitored.
Although skin blood flow was not measured in the present study, it seems unlikely that the large regional differences observed in Tsk across the body were independently caused by differences in blood flow distribution. Rather, it seems more likely that heterogeneity of regional Tsk was a result of intermittent and non-uniform RW heat output across the body surface. Indeed, Fic et al.7 recently reported a mathematically modeled map of neonatal skin temperatures under a RW and estimated a ‘very non-uniform temperature distribution’. Although their predicted skin temperatures far exceeded those directly measured in the present study, the caudal-to-rostral temperature gradient observed directly supports their primary conclusions, as do the peak skin temperatures measured on the forehead and chest (Figure 2). The maximum caudal-to-rostral temperature difference observed presently was ~6 °C, which is more than four times greater than the foot-to-head temperature difference previously reported in a similarly aged patient population nursed in incubators.25 It is also possible that this difference would be further exacerbated if head-up tilt were applied to the infant bed to improve oxygenation.26 Fic et al.7 proposed using a blanket with a high conductivity to create a more even temperature distribution across the skin of a neonate nursed under a RW. Our findings potentially support this notion, however, it is clear that without a blanket, skin temperature must be monitored elsewhere on the body to ensure that extreme temperatures do not occur. Given that abdominal or forehead skin temperatures were the highest and foot skin temperatures were the lowest (Figure 2), continuous measurement of these regions, if possible, would be optimal.
Limitations of the present study include the fact that only six different skin temperature sites were measured. Although most major body regions were evaluated, the skin temperature of the hand and calf were not measured. All temperature measurements were taken on one side of the body (right) and were therefore assumed to be representative of the other side of the body; given that the radiant flux profile of a RW is the same in the lateral plane,7 similar values would have likely been observed on the left hand side of the body. All skin temperature probes were covered with the same standard reflective shield as the Tfeedback probe to avoid a direct influence of radiant heat output on the probe itself. However, this reflective covering may lead to a small underestimation of real skin temperature,27 therefore, the actual skin temperatures in Figure 2 may be slightly higher. A final limitation is that all participants in the present study were sedated with fentanyl and whether the present findings are applicable to other sedatives is unclear.
In conclusion, the present study raises two points of concern. First, the feedback provided from a single skin temperature is unlikely to be a reliable representation of true deep core temperature (for example, Tre). Second, skin temperature values across the body are non-uniform irrespective of Tfeedback, with up to a ~6 °C difference observed between the feet and the forehead/torso, which is four times greater than previously reported in an incubator.25 Our data demonstrate that the management of neonatal body temperature under a RW device still requires a high level of attention from caregivers, and modifications to care, such as the additional monitoring of other skin surface temperatures and deep core temperature may be necessary to ensure optimal thermal management.
References
Sherman TI, Greenspan JS, St Clair N, Touch SM, Shaffer TH . Optimizing the neonatal thermal environment. Neonatal Netw 2006; 25: 251–260.
George G, Mishra S . Routine axillary temperature monitoring in neonates cared under radiant warmer - is it necessary? Indian J Pediatr 2009; 76: 1281–1282.
Bell EF . Infant incubators and radiant warmers. Early Hum Dev 1983; 8: 351–375.
Meyer MP, Payton MJ, Salmon A, Hutchinson C, de Klerk A . A clinical comparison of radiant warmer and incubator care for preterm infants from birth to 1800 grams. Pediatrics 2001; 108: 395–401.
Trevisanuto D, Coretti I, Doglioni N, Udilano A, Cavallin F, Zanardo V . Effective temperature under radiant infant warmer: does the device make a difference? Resuscitation 2011; 82: 720–723.
Molgat-Seon Y, Daboval T, Chou S, Jay O . Assessing neonatal heat balance and physiological strain in newborn infants nursed under radiant warmers in intensive care with fentanyl sedation. Eur J Appl Physiol 2014; 114: 2539–2549.
Fic AM, Ingham DB, Ginalski MK, Nowak AJ, Wrobel LC . Modelling and optimisation of the operation of a radiant warmer. Med Eng Phys 2014; 36: 81–87.
Molgat-Seon Y, Daboval T, Chou S, Jay O . Accidental overheating of a newborn under an infant radiant warmer: a lesson for future use. J Perinatol 2013; 33: 738–739.
Gomella TL, Cunningham MD, Eyal FG, Zenk KE . Neonatology: Management, Procedures, On-call Problems, Diseases, and Drugs. Appleton & Lange: Stamford, CT, USA, 1999.
Schafer D, Boogaart S, Johnson L, Keezel C, Ruperts L, Vander Laan KJ . Comparison of neonatal skin sensor temperatures with axillary temperature: does skin sensor placement really matter? Adv Neonatal Care 2014; 14: 52–60.
Mayfield SR, Bhatia J, Nakamura KT, Rios GR, Bell EF . Temperature measurement in term and preterm neonates. J Pediatr 1984; 104: 271–275.
Karlsson H . Skin to skin care: heat balance. Arch Dis Child Fetal Neonatal Ed 1996; 75: F130–F132.
Buntain WL, Pregler M, O'Brien PC, Lynn HB . Axillary versus rectal temperature: a comparative study. J La State Med Soc 1977; 129: 5–8.
Bland JM, Altman DG . Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat 2007; 17: 571–582.
Krouwer JS . Why Bland–Altman plots should use X, not (Y+ X)/2 when X is a reference method. Stat Med 2008; 27: 778–780.
Kimberger O, Thell R, Schuh M, Koch J, Sessler DI, Kurz A . Accuracy and precision of a novel non-invasive core thermometer. Br J Anaesth 2009; 103: 226–231.
Suleman M-I, Doufas AG, Akça O, Ducharme M, Sessler DI . Insufficiency in a new temporal-artery thermometer for adult and pediatric patients. Anesth Analg 2002; 95: 67–71.
Kimberger O, Cohen D, Illievich U, Lenhardt R . Temporal artery versus bladder thermometry during perioperative and intensive care unit monitoring. Anesth Analg 2007; 105: 1042–1047.
Moran JL, Peter JV, Solomon PJ, Grealy B, Smith T, Ashforth W et al. Tympanic temperature measurements: Are they reliable in the critically ill? A clinical study of measures of agreement*. Crit Care Med 2007; 35: 155–164.
Giuffre M, Heidenreich T, Carney-Gersten P, Dorsch JA, Heidenreich E . The relationship between axillary and core body temperature measurements. Appl Nurs Res 1990; 3: 52–55.
Maxton FJ, Justin L, Gillies D . Estimating core temperature in infants and children after cardiac surgery: a comparison of six methods. J Adv Nurs 2004; 45: 214–222.
Molgat-Seon Y . Quantifying heat balance components in neonates nursed under radiant warmers during intensive care. 2012. MSc Thesis, University of Ottawa: Ottawa, ON, Canada.
Kroth J, Weidlich K, Hiedl S, Nussbaum C, Christ F, Genzel-Boroviczény O . Functional vessel density in the first month of life in preterm neonates. Pediatr Res 2008; 64: 567–571.
Toon MH, Maybauer DM, Arceneaux LL, Fraser JF, Meyer W, Runge A et al. Children with burn injuries—assessment of trauma, neglect, violence and abuse. J Inj Violence Res 2011; 3: 98–110.
Karlsson H, Olegard R, Nilsson K . Regional skin temperature, heat flow and conductance in preterm neonates nursed in low and in neutral environmental temperature. Acta Paediatr 1996; 85: 81–87.
Thoresen M, Cowan F, Whitelaw A . Effect of tilting on oxygenation in newborn infants. Arch Dis Child 1988; 63: 315–317.
LeBlanc MH, Edwards NK . Artifacts in the measurement of skin temperature under infant radiant warmers. Ann Biomed Eng 1985; 13: 443–450.
Haycock GB, Schwartz GJ, Wisotsky DH . Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 1978; 93: 62–66.
Acknowledgements
This research was supported by a Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery Grant (Grant Holder: OJ) as well as by a Children’s Hospital of Eastern Ontario Department of Pediatric Surgery Seed Fund Grant (Grant Holder: SC). YMS was supported by a University of Ottawa Master’s Scholarship. We wish to thank the volunteers for their co-operation and the CHEO NICU Nursing staff for their assistance during data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chaseling, G., Molgat-Seon, Y., Daboval, T. et al. Body temperature mapping in critically ill newborn infants nursed under radiant warmers during intensive care. J Perinatol 36, 540–543 (2016). https://doi.org/10.1038/jp.2016.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.16