Abstract
Core temperature (TCore) monitoring is essential in intensive care medicine. Bladder temperature is the standard of care in many institutions, but not possible in all patients. We therefore compared core temperature measured with a zero-heat flux thermometer (TZHF) and with a bladder catheter (TBladder) against blood temperature (TBlood) as a gold standard in 50 critically ill patients in a prospective, observational study. Every 30 min TBlood, TBladder and TZHF were documented simultaneously. Bland–Altman statistics were used for interpretation. 7018 pairs of measurements for the comparison of TBlood with TZHF and 7265 pairs of measurements for the comparison of TBlood with TBladder could be used. TBladder represented TBlood more accurate than TZHF. In the Bland Altman analyses the bias was smaller (0.05 °C vs. − 0.12 °C) and limits of agreement were narrower (0.64 °C to − 0.54 °C vs. 0.51 °C to – 0.76 °C), but not in clinically meaningful amounts. In conclusion the results for zero-heat-flux and bladder temperatures were virtually identical within about a tenth of a degree, although TZHF tended to underestimate TBlood. Therefore, either is suitable for clinical use.
German Clinical Trials Register, DRKS00015482, Registered on 20th September 2018, http://apps.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00015482.
Similar content being viewed by others
Introduction
Core temperature monitoring is one of the essential monitoring modalities in intensive care medicine and temperatures below or above the normal core temperature can be observed frequently. Hypothermia at admission of surgical patients is associated with many adverse outcomes like coagulopathy1, increased bleeding2, and higher transfusion rates3 as well as increased surgical site infections4,5 and in some studies even with mortality3,6,7,8,9. However, fever is observed much more frequently in critically ill patients and warrants a diagnostic workup to determine the presence of potential infections10. The ideal temperature measurement method should provide reliable, reproducible values safely and conveniently10. Additionally, the device should be small, easy to use, comfortable, fast, continuous, noninvasive, low energy consuming and affordable11. In many institutions bladder temperature (TBladder) is the standard of care because it is accurate, provides continuous readings, stable measurements regardless of urine flow rate and stays securely in place even during positioning of the patient10. Normally TBladder monitoring adds no additional invasiveness to the standard monitoring, because bladder catheters are nearly always used in critically ill patients.
However, not every patient meets the criteria for a bladder catheter. Patients with acute or chronic renal failure with anuria, after cystectomy or awake patients do not need a bladder catheter. With no indication for using a bladder catheter the use is associated with an unnecessary risk of nosocomial infection. Further on, patients that need irrigation of the bladder because of bleeding will not be appropriate for measurement of TBladder. In these situation an alternative approach is needed. Especially in alert patients a non-invasive monitor is helpful. Unfortunately, many of these methods are not very reliable12. A better alternative might be a zero-heat flux (ZHF) thermometer that has been proven to be reliable in surgical patients13,14,15,16. In general, zero-heat-flux thermometers consist of a thermal insulator adjacent to the skin that is covered by a servo-controlled electric heater. The heater is used to eliminate the flow of heat through the insulator, so that the temperature of the heater and skin temperatures are equal13. A validation study in critically ill patients is important because in patients undergoing surgery the most common thermal problem is perioperative hypothermia whereas in critically ill patients it is fever. However, to date no large comparison in critically ill patients has been performed with an accepted gold standard like blood temperature. There is only one study available that has included a small number of patients with blood temperature as a reference method26.
The aim of this study was to compare TCore measured with a ZHF-thermometer (TZHF) and with TBladder against a gold standard TBlood measured in the iliac artery or pulmonary artery to determine if the new ZHF-thermometer is more accurate than TBladder.
Methods
The current prospective clinical study was conducted in accordance with the declaration of Helsinki at the University Hospital of Göttingen, Germany, after obtaining local ethics committee approval (Ethics committee of the University Medicine Göttingen, Application number: 13/05/18) for the experimental protocol and registration on German Clinical Trials Register (DRKS00015482). According to the approval of the local ethical committee we used deferred (proxy) consent in emergency critical care research17 as the study was totally non-invasive and observative. If patients were able to give informed written consent this consent was used. If informed proxy consent was necessary, it was given in written form of the proxy. We did not exclude patients who did not recover and died during their hospital stay. The local ethics committee had approved this procedure. The article adheres to the STROBE guidelines18.
Critically ill adult patients already having a bladder catheter with a temperature probe and an arterial catheter with a temperature probe placed in the iliac artery (Pulsiocath Arterial Thermodilution Catheter 5F; Pulsion Medical Systems AG, Munich, Germany) or a pulmonary artery catheter (Arrow Hands-Off Thermodilution catheter 7F; Arrow International, Athlone, Irland) in place were included in this this study. The only exclusion criteria were pregnancy and refusal to take part in the study.
In all patients, core temperature was measured additionally with a single use, continuous, non-invasive ZHF-thermometer (3 M SpotOn Temperature Monitoring System, 3 M, St. Paul, MN, USA) attached to the lateral forehead of the patients.
Then every 30 min TCore measured by TBlood, TBladder and the ZHF-thermometer were documented at the same time points until the patient lost the TBlood sensor or TBladder sensor, left the ICU or at least after 5 days. If data of a temperature source were missing the couple of data was not used for comparison. In addition to the temperature data age, weight, height, sex and medical diagnosis at admission to the Intensive Care Unit (ICU) were documented.
As a primary statistical method Bland–Altman statistics were used for interpretation of accuracy (bias = mean difference between methods) and precision (limits of agreement = 1.96 standard deviation) using the Bland and Altman random effects method for repeated measures data adjusted for unequal numbers of measurements per patient19. Additionally, we calculated the proportion of all differences that were within ± 0.5 °C or ± 1 °C of TBlood.
For each of the two measurement modalities sensitivity, specificity, positive and negative predictive values for the detection of hypothermia and fever were calculated. Hypothermia was defined as a TBlood < 36 °C and fever was defined as TBlood > 38.3 °C10.
Additionally, we performed an error grid analysis20 to determine if some measurement differences would lead to wrong clinical decisions. The Zones were defined as follows:
Zone A begins with an area of a ± 0.5 °C error on either side of a perfectly accurate measurement between TBlood and the temperature measured by TZHF or TBladder. Measurement errors smaller than ± 0.5 °C are considered by most authors as clinically not relevant. In addition, if both measurements indicate hypothermia < 36 °C or fever > 38.3 °C the absolute error is considered to be clinically irrelevant because the same treatment or diagnostic workup will be initiated.
Zone B describes the zone where measurement errors are > 0.5 °C but this will not result in a clinical wrong decision. E.g. if TBlood is 36.5 °C and TZHF shows a temperature of 37.4 °C both temperatures will not lead to active warming therapy or a diagnostic workup for infection.
In contrast Zone C indicates errors larger than 0.5 °C that will lead to wrong clinical decisions and may do harm to the patient. e.g. if TBlood is 34 °C and TZHF shows 37 °C the patient will not receive active warming although this would be indicated.
Results
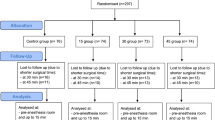
55 potentially eligible patients were screened. Three patients could not be enrolled because we could not obtain proxy consent and two patients were not included due to technical problems. The remaining 50 patients were enrolled. 36 patients (72%) were male, 14 (28%) were female. Mean age was 61.9 (± 16.8) years, mean height was 1.75 (± 0.07) m, mean weight was 86.4 (± 36.3) kg resulting in a mean body mass index of 28.2 (± 11.3) kg/m2. Of these patients 16 were suffering from sepsis, 18 patients had neurologic injury (subarachnoid hemorrhage, intracerebral hemorrhage), 6 patients had trauma, 4 patients had respiratory failure, 2 patients had accidental hypothermia, 3 patients had cardiac surgery, and 1 patient had visceral surgery. Of all 50 patients 49 had an arterial catheter with a temperature probe placed in the iliac artery and one patient had a pulmonary artery catheter with temperature probe. No patient was excluded from the study after enrolment.
Globally 3970.5 h were recorded. 7665 TBlood values, 7086 values of TZHF and 7358 Tbladder values were documented. 276 TBlood values, 855 values of the ZHF-thermometer and 583 TBladder values were missing. The major reason for missing values was a disconnection of the temperature probes for transportation of the patient to the CT, OR, neuroradiology suite, or to the cardiac catheter lab. After these procedures the devices were often not reconnected immediately. Only 17 temperature values of TBladder and 16 values of TZHF were missing due to technical problems. 12 values below 30 °C could not be recorded by the ZHF-thermometer because the device did not give a reading at these low temperatures.
This resulted in 7018 pairs of measurements for the comparison of TBlood with TZHF and 7265 pairs of measurements for the comparison of TBlood with TBladder.
In 530 measurements TBlood was < 36 °C, in 6665 measurements TBlood was 36–38.3 °C and in 470 measurements TBlood was > 38.3 °C.
Bland Altman analysis
Bias between TZHF and TBlood was − 0.12 °C with an upper limit of agreement of 0.51 °C and a lower limit of agreement of − 0.76 °C (Fig. 1). Bias between TBladder and TBlood was 0.05 °C with an upper limit of agreement of 0.64 °C and a lower limit of agreement of − 0.54 °C (Fig. 2).
Proportion of differences within ± 0.5 °C and ± 1 °C
The proportion of differences within ± 0.5 °C of TBlood was 90.98% for TZHF and 95.99% for TBladder and the proportion of differences within ± 1.0 °C of TBlood was 98.99% for TZHF and 99.01% for TBladder.
Sensitivity, specificity, positive and negative predictive values
The calculated sensitivity, specificity, positive and negative predictive values for the detection of hypothermia and fever are shown in Table 1.
Error grid analysis
Error grid analysis showed that 91.6% of all TZHF measurements were clinically not different from TBlood, or would still lead to the same treatment or diagnostic workup. In 6.2% measurement errors were > 0.5 °C, but the result would not lead to a clinical wrong decision. Only 2.2% of the measurements would lead to wrong clinical decisions (Fig. 3). Error grid analysis of TBladder showed that 96.3% of all measurements were clinically not different from TBlood or would still lead the same treatment or diagnostic workup. In 2.4% measurement errors were > 0.5 °C but this would not result in a clinical wrong decision. Only 1.3% of the measurements would lead to wrong clinical decisions (Fig. 4).
Adverse events
The ZHF-thermometer sensors were well tolerated in all patients and no burn or skin reaction was observed during the study period.
Discussion
In this study with critically ill patients, TBladder represented TBlood more accurate than TZHF. In the Bland Altman analyses the bias was smaller and limits of agreement were narrower. The proportion of differences within ± 0.5 °C of TBlood were higher, and there were less values in Zone B and C of the error grid analysis. In addition, the ZHF thermometer failed to record core temperatures below 30 °C. However, compared to the published results for other non-invasive thermometers like infrared tympanic membrane thermometers, temporal artery thermometers, or axillary thermometers12 the ZHF-thermometer is more accurate.
Interpretation of our results
The results of the Bland Altman analysis of TBladder were comparable to the results that were obtained by Nierman21 and slightly different from the results of Lefrant et al.22 who observed a bias of − 0.21 °C and more narrow limits of agreement of ± 0.20 °C. In general, the high level of accuracy of TBladder is remarkable because oliguria, that is very frequent in ICU patients, reduces the accuracy of TBladder measurements in operative patients23,24. On the other hand, in critically ill patients, oliguria does not seem to influence the accuracy of bladder temperature very much10.
The results of the Bland Altman analysis of the ZHF-thermometer were a slightly better than the results that were obtained by Eshraghi et al.13 before and after cardiopulmonary bypass, and in the same range as found by Mäkinen et al.15 during cardiac surgery when the patients were off cardiopulmonary bypass. During surgery with slow temperature changes Boisson et al.14 could obtain better results with a bias to TBlood of − 0.1 °C with limits of agreement of ± 0.4 °C.
The proportion of differences within ± 0.5 °C of TBlood was 84% in the study of Eshraghi13 and 94% in the study of Boisson14. Our results of 91% are also in that range. Other studies that have evaluated the ZHF-thermometer in critically ill patients did not compare it to a gold standard and are therefore of limited value for the comparison with our results25,26.
The question is, if the accuracy of the ZHF-thermometer is still good enough to be used in ICU. Many studies that compare new temperature monitoring devices with a gold standard use a definition that the combined inaccuracy (bias and limits of agreement) should be smaller than 0.5 °C27 to be accurate enough. In our opinion this objective is very high and most of the studies that have investigated new non-invasive thermometers13,28,29 did not find an accuracy that met this criterion. Still they came to the conclusion that the new devices agree sufficiently enough for clinical practice13,28,29.
Another possibility is to look at the proportion of differences within the range of ± 0.5 °C of the TBlood. In our study 91% of all measurement values of the ZHF-thermometer were within the range of ± 0.5 °C of TBlood and 99% were within the range of ± 1 °C. That seems to be acceptable.
Another interesting way of interpreting the results is the error grid analysis20. In this analysis 91.6% of the values of the ZHF-thermometer lead to the right clinical decision and only 2.2% of the measured values would lead to wrong clinical decisions. This seems to be sufficient, especially because TCore changes do not require an immediate change in therapy in the next minutes. However, it has been argued, that no single measurement value should be in Zone C as this will lead to wrong clinical decisions20. This seems to be very demanding. If we would accept this, methods like non-invasive blood pressure measurement or pulse oximetry would have to be abandoned immediately.
Limitations of the study
In most studies comparing temperature measurement devices there are many data pairs per subject and the number of data pairs per patient are not equal. This can induce random effects because there are independent influences of the different patients and there are influences of time in each individual patient. This influence is not totally independent. To account for this effect, we have used the Bland and Altman random effects method for repeated measures data adjusted for unequal numbers of measurements per patient19.
A potential limitation of the methods used is the use of error grid analysis. This method has not been used for the comparison of temperature measurement devices before. Error grid analysis is highly dependent on the zones, which can are by definition arbitrarily defined. In this study the zones were defined by the authors a priori using published and well accepted definitions. Zone A was defined as an area of a ± 0.5 °C error on either side of a perfectly accurate measurement between TBlood and the TZHF or TBladder because measurement errors smaller than ± 0.5 °C are considered by most authors as clinically not relevant. In addition, if both measurements indicate hypothermia < 36 °C or fever > 38.3 °C the absolute error is considered to be clinically irrelevant because the same treatment or diagnostic workup will be initiated. It can be argued that there is a clinically relevant difference between 35.0 °C and 26 °C. This would still lead to a data point that is in the Zone A. However, it is extremely difficult to define thresholds for this situation. In addition, we did not observe this.
Other potential limitations of our study are that we have studied a relatively small population with only 50 patients. However, in average every patient was monitored more than 3.3 days, resulting in an average of about 140 measurement points per patient.
Another potential limitation is that we have studied a mixed ICU patient collective. This may also be seen as an advantage because we have measured different patients in different critically ill states and with different influences like renal replacement therapy (RRT) or Extracorporeal Membrane Oxygenation (ECMO). Patients undergoing targeted temperature management after cardiopulmonary resuscitation which might be an interesting and challenging patient cohort in which TCore measurement is of utmost importance are missing in our collective. This may be a limitation to the generalizability of the study results.
In some of our patients the gold standard blood temperature may be distorted by the rapid infusion of unwarmed fluids or extracorporeal devices like RRT or ECMO. It is well known that a rapid infusion of unwarmed or cold fluids can lower blood temperature temporarily. This effect is typically used for the measurement cardiac output with a pulmonary artery catheter. This effect varies depending on the temperature, amount, and rate of the fluid given. Initiation of RRT also temporarily changes blood temperature to a small amount but a stable running RRT does not lead to changes in blood temperature. The same is probably true for ECMO. Infusion of intravenous fluids or RRT are typical measures in ICU and it is not possible to exclude patients that need intravenous fluids. In our patient group 17 patients had RRT and 2 patients had ECMO. This may have contributed to the observed inaccuracy of the ZHF-thermometer and TBladder. Another potential problem may be that the analogue data transfer from the ZHF-thermometer to the general ICU monitoring may have introduced an additional error.
We did also not observe many measurements for temperature above 39 °C. Therefore, it is not possible to make any conclusions about the accuracy the devices in these extremely high temperature range.
The use of vasopressor therapy and especially the use of high dose vasopressor therapy may also influence the accuracy of the ZHF-thermometer. Unfortunately, we did not look at this potential source of inaccuracy. This might be investigated in another study.
Also we did not measure the urine output of our patients, therefore a correlation to accuracy of TBladder is impossible.
Some studies have used more complex statistical methods29 like population analysis30. However, very often these complex analyses do not add very much new information about the accuracy of the studied devices. We included sensitivity, specificity, positive and negative predictive values for the detection of hypothermia and fever for both methods because this has not been done yet. We also included an error grid analysis because this kind of analysis may be clinically useful although the definition of the three zones in that error grid analysis can be discussed.
Conclusion
In conclusion the results for zero-heat-flux and bladder temperatures were virtually identical within about a tenth of a degree, although TZHF tended to underestimate TBlood. Therefore, either is suitable for clinical use and can be used if bladder temperature is not available.
Data availability
The datasets used for the analysis in the current study are available from the corresponding author on reasonable request.
References
Rohrer, M. J. & Natale, A. M. Effect of hypothermia on the coagulation cascade. Crit. Care Med. 20, 1402–1405 (1992).
Hohn, L. et al. Benefits from intraoperative skin surface warming in cardiac surgical patients. Br. J. Anaesth. 80, 318–323 (1998).
Insler, S. R. et al. Association between postoperative hypothermia and adverse outcome after coronary artery bypass surgery. Ann. Thorac. Surg. 70, 175–181 (2000).
Kurz, A., Sessler, D. I. & Lenhardt, R. Perioperative normothermia to reduce the incidence of surgical- wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N. Engl. J. Med. 334, 1209–1215 (1996).
Melling, A. C., Ali, B., Scott, E. M. & Leaper, D. J. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet 358, 876–880 (2001).
Scott, A. V. et al. Compliance with surgical care improvement Project for body temperature management (SCIP Inf-10) is associated with improved clinical outcomes. Anesthesiology 123, 116–125 (2015).
Billeter, A. T. et al. Unintentional perioperative hypothermia is associated with severe complications and high mortality in elective operations. Surgery. 156, 1245–1252 (2014).
Karalapillai, D. et al. Inadvertent hypothermia and mortality in postoperative intensive care patients: retrospective audit of 5050 patients. Anaesthesia. 64, 968–972 (2009).
Niven, D. J., Stelfox, H. T. & Laupland, K. B. Hypothermia in adult ICUs: changing incidence but persistent risk factor for mortality. J. Intensive Care Med. 31, 529–536 (2014).
O’Grady, N. P. et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit. Care Med. 36, 1330–1349 (2008).
Wartzek, T., Mühlsteff, J. & Imhoff, M. Temperature measurement. Biomed. Tech. (Berl) 56, 241–257 (2011).
Niven, D. J. et al. Accuracy of peripheral thermometers for estimating temperature: a systematic review and meta-analysis. Ann. Intern. Med. 163, 768–777 (2015).
Eshraghi, Y. et al. An evaluation of a zero-heat-flux cutaneous thermometer in cardiac surgical patients. Anesth. Analg. 119, 543–549 (2014).
Boisson, M. et al. Intra-operative cutaneous temperature monitoring with zero-heat-flux technique (3M SpotOn) in comparison with oesophageal and arterial temperature: a prospective observational study. Eur. J. Anaesthesiol. 35, 825–830 (2018).
Mäkinen, M. T. et al. Novel zero-heat-flux deep body temperature measurement in lower extremity vascular and cardiac surgery. J. Cardiothorac. Vasc. Anesth. 30, 973–978 (2016).
Pesonen, E. et al. The focus of temperature monitoring with zero-heat-flux technology (3M Bair-Hugger): a clinical study with patients undergoing craniotomy. J. Clin. Monit. Comput. 33, 917–923 (2019).
Jansen, T. C., Kompanje, E. J. & Bakker, J. Deferred proxy consent in emergency critical care research: ethically valid and practically feasible. Crit. Care Med. 37, S65–S68 (2009).
von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370, 1453–1457 (2007).
Bland, J. M. & Altman, D. G. Agreement between methods of measurement with multiple observations per individual. J. Biopharm. Stat. 17, 571–582 (2007).
Morey, T. E., Gravenstein, N. & Rice, M. J. Let’s think clinically instead of mathematically about device accuracy. Anesth. Analg. 113, 89–91 (2011).
Nierman, D. M. Core temperature measurement in the intensive care unit. Crit. Care Med. 19, 818–823 (1991).
Lefrant, J.-Y. et al. Temperature measurement in intensive care patients: comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med. 29, 414–418 (2003).
Sato, H. et al. Urinary bladder and oesophageal temperatures correlate better in patients with high rather than low urinary flow rates during non-cardiac surgery. Eur. J. Anaesthesiol. 25, 805–809 (2008).
Horrow, J. C. & Rosenberg, H. Does urinary catheter temperature reflect core temperature during cardiac surgery?. Anesthesiology 69, 986–879 (1988).
Schell-Chaple, H. M., Liu, K. D., Matthay, M. A. & Puntillo, K. A. Rectal and bladder temperatures vs forehead core temperatures measured with SpotOn monitoring system. Am. J. Crit. Care. 27, 43–50 (2018).
Dahyot-Fizelier, C. et al. Accuracy of zero-heat-flux cutaneous temperature in intensive care adults. Crit. Care Med. 45, e715–e717 (2017).
Sessler, D. I. Temperature monitoring and perioperative thermoregulation. Anesthesiology 109, 318–338 (2008).
Kimberger, O. et al. Accuracy and precision of a novel non-invasive core thermometer. Br. J. Anaesth. 103, 226–231 (2009).
Kimberger, O. et al. The accuracy of a disposable noninvasive core thermometer. Can. J. Anaesth. 60, 1190–1196 (2013).
Soehle, M., Dehne, H., Hoeft, A. & Zenker, S. Accuracy of the non-invasive Tcore temperature monitoring system to measure body core temperature in abdominal surgery. J. Clin. Monit. Comput. 34, 1361–1367 (2020).
Acknowledgements
We would like to thank the nursing staff for supporting the study.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was conducted with departmental funding only. No equipment was loaned or supplied by a company.
Author information
Authors and Affiliations
Contributions
A.B., A.F., T.P., and I.F.B. designed the study. A.B., A.F., D.H. and K.M. managed data and its quality. A.B., A.F., and I.F.B. performed the statistical analysis. All authors participated in the data interpretation. A.B. drafted the manuscript. A.F., D.H., T.P., I.F.B. and K.M. contributed substantially to its revision. All authors read the manuscript carefully and approved the final version.
Corresponding author
Ethics declarations
Competing interests
Prof. Dr. A. Bräuer is a member of the advisory board of 3 M Europe and has received payments from 3 M Germany, 3 M Europe, 3 M Asia Pacific Pte Ltd. for consultancy work. PD Dr. T. Perl T has received consulting honorary from The 37Company the Netherlands and Barkey, Germany. The other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bräuer, A., Fazliu, A., Perl, T. et al. Accuracy of zero-heat-flux thermometry and bladder temperature measurement in critically ill patients. Sci Rep 10, 21746 (2020). https://doi.org/10.1038/s41598-020-78753-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78753-w
This article is cited by
-
Accuracy of a zero-heat-flux thermometer in cardiac surgery, a prospective, multicentre, method comparison study
Scientific Reports (2024)
-
Free-living core body temperature monitoring using a wrist-worn sensor after COVID-19 booster vaccination: a pilot study
BioMedical Engineering OnLine (2023)
-
Accuracy of non-invasive sensors measuring core body temperature in cardiac surgery ICU patients – results from a monocentric prospective observational study
Journal of Clinical Monitoring and Computing (2023)
-
Evaluation of the Temple Touch Pro™ noninvasive core-temperature monitoring system in 100 adults under general anesthesia: a prospective comparison with esophageal temperature
Journal of Clinical Monitoring and Computing (2023)
-
Sustained exercise load by young adult females while wearing surgical mask raises core body temperature measured with zero-heat-flux thermometer
International Journal of Biometeorology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.