Abstract
Pulmonary artery thrombus is a rarely reported complication in premature neonates. The management of life-threatening thrombotic events in neonates is controversial, especially regarding the use of thrombolytics versus anticoagulation alone for treatment. We report a case of a premature neonate with symptomatic pulmonary artery thrombus treated with recombinant tissue plasminogen activator who survived without bleeding complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Biss TT, Brandao LR, Kahr WH, Chan AK, Williams S . Clinical features and outcome of pulmonary embolism in children. Br J Haematol 2008; 142 (5): 808–818.
Sawyer T, Antle A, Studer M, Thompson M, Perry S, Mahnke CB . Neonatal pulmonary artery thrombosis presenting as persistent pulmonary hypertension of the newborn. Pediatr Cardiol 2009; 30 (4): 520–522.
Kenny D, Tsai-Goodman B . Neonatal arterial thrombus mimicking congenital heart disease. Arch Dis Child Fetal Neonatal Ed 2007; 92 (1): F59–F61.
Thornburg C, Pipe S . Neonatal thromboembolic emergencies. Semin Fetal Neonatal Med 2006; 11 (3): 198–206.
Coleman MM, Spear ML, Finkelstein M, Leef KH, Pearlman SA, Chien C et al. Short-term use of umbilical artery catheters may not be associated with increased risk for thrombosis. Pediatrics 2004; 113: 770–774.
Veldman A, Nold MF, Michel-Behnke I . Thrombosis in the critically ill neonate: incidence, diagnosis, and management. Vasc Health Risk Manag 2008; 4: 1337–1348.
Kuhle S, Massicotte P, Chan A, Adams M, Abdolell M, de Veber G et al. Systemic thromboembolism in children: data from the 1-800-NO-CLOTS Consultation Service. Thromb Haemost 2004; 92: 722–728.
Monagle P, Chalmers E, Chan A, DeVeber G, Kirkham F, Massicotte P et al. Antithrombotic therapy in neonates and children: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133 (Suppl 6): 887S–968S.
Anderson B, Urs P, Tudehope D, Ward C . The use of recombinant tissue plasminogen activator in the management of infective intracardiac thrombi in pre-term infants with thrombocytopaenia. J Paediatr Child Health 2009; 45: 598–601.
Farnoux C, Camard O, Pinquier D, Hurtaud-Roux MF, Sebag G, Schlegel N et al. Recombinant tissue-type plasminogen activator therapy of thrombosis in 16 neonates. J Pediatr 1998; 133: 137–140.
Hartmann J, Hussein A, Trowitzsch E, Becker J, Hennecke KH . Treatment of neonatal thrombus formation with recombinant tissue plasminogen activator: six years experience and review of the literature. Arch Dis Child Fetal Neonatal Ed 2001; 85: F18–F22.
John CM, Harkensee C . Thrombolytic agents for arterial and venous thromboses in neonates. Cochrane Database Syst Rev 2005; CD004342.
Acknowledgements
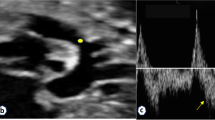
We thank Dr Christoph Hornik and Dr Piers C Barker for providing echocardiogram images and videos.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Journal of Perinatology website
Supplementary information
Rights and permissions
About this article
Cite this article
DeMeo, S., Sherwood, A., Hornik, C. et al. Pulmonary artery thrombus in a premature neonate treated with recombinant tissue plasminogen activator. J Perinatol 34, 569–571 (2014). https://doi.org/10.1038/jp.2014.34
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2014.34
Keywords
This article is cited by
-
Spontaneous pulmonary artery thrombus in a neonate
The Egyptian Heart Journal (2021)