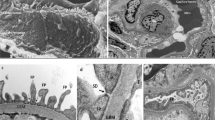
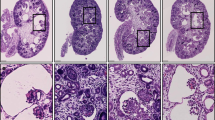
Abstract
Two siblings with a severe multiorgan polycystic disease presenting in the neonatal period were identified. Their genetic testing identified compound heterozygous NPHP3 gene mutations, parents being heterozygous carriers. The mutations included a splice-site (c.958-2A>G) and a missense mutation (c.2342G>A; p.G781D), both being extremely rare. NPHP3 encodes for nephrocystin 3 present on the cilia-centrosome complex. We hypothesize that these mutations lead to defective cilia-based signaling, required for normal development of the renal, pancreatic, biliary and portal system. This report outlines a rare neonatal ciliopathy presentation of NPHP3 mutations leading to severe multiorgan failure in two siblings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hildebrandt F, Benzing T, Katsanis N . Ciliopathies. N Engl J Med 2011; 364 (16): 1533–1543.
Barr M, Sternberg P . A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature 1999; 401 (6751): 386–389.
Filegauf M, Benzing T, Omran H . When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol 2007; 8 (11): 880–893.
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P et al. A method and server for predicting damaging missense mutations. Nat Methods 2010; 7 (4): 248–249.
Ng PC, Henikoff S . Predicting deleterious amino acid substitutions. Genome Res 2001; 11 (5): 863–874.
Ng PC, Henikoff S . SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res 2003; 31 (13): 3812–3814.
Schwarz JM, Rodelsperger C, Schuelke M, Seelow D . MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 2010; 7 (8): 575–576.
Hildebrandt F, Zhou W . Nephronophthisis-associated ciliopathies. J Am Soc Nephrol 2007; 18 (6): 1855–1871.
Bergmann C, Fliegauf M, Bruchle N, Frank V, Olbrich H, Kirschner J et al. Loss of nephrocystin-3 function can cause embryonic lethality, Mecker-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet 2008; 82 (4): 959–970.
Bandano J, Mitsuma N, Beales P, Katsanis N . The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet 2006; 7: 125–148.
Olbrich H, Fliegauf M, Hoefele J, Kispert A, Otto E, Volz A et al. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat Genet 2003; 34 (4): 455–459.
Omran H, Haffner K, Burth S, Fernandez C, Fargier B, Villaquiran A et al. Human adolescent nephronophthisis: gene locus synteny with polycystic kidney disease in pcy mice. J Am Soc Nephrol 2001; 12 (1): 107–113.
Simpson M, Cross H, Cross L, Helmuth M, Crosby A . Lethal cystic kidney disease in Amish neonates associated with homozygous nonsense mutation of NPHP3. Am J Kidney Dis 2009; 53 (5): 790–795.
Halbritter J, Diaz K, Chaki M, Porath J, Tarrier B, Fu C et al. High-throughput mutation analysis in patients with a nephronophthisis-associated ciliopathy applying multiplexed barcoded array PCR amplification and next-generation sequencing. J Med Genet 2012; 49 (12): 756–767.
Halbritter J, Porath J, Diaz J, Braun D, Kohl S, Chaki M et al. Identification of 99 novel mutations in a worldwide cohort of 1056 patients with nephronophthisis-related ciliopathy. Hum Genet 2013; 132: 865–884.
Acknowledgements
This work was funded by a grant from The Manton Center for Orphan Disease Research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Leeman, K., Dobson, L., Towne, M. et al. NPHP3 mutations are associated with neonatal onset multiorgan polycystic disease in two siblings. J Perinatol 34, 410–411 (2014). https://doi.org/10.1038/jp.2014.20
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2014.20