Abstract
Purpose
To investigate the prevalence of biallelic PKD1 and PKD2 variants underlying very early onset (VEO) polycystic kidney disease (PKD) in a large international pediatric cohort referred for clinical indications over a 10-year period (2010–2020).
Methods
All samples were tested by Sanger sequencing and multiplex ligation-dependent probe amplification (MLPA) of PKD1 and PKD2 genes and/or a next-generation sequencing panel of 15 additional cystic genes including PKHD1 and HNF1B. Two patients underwent exome or genome sequencing.
Results
Likely causative PKD1 or PKD2 variants were detected in 30 infants with PKD-VEO, 16 of whom presented in utero. Twenty-one of 30 (70%) had two variants with biallelic in trans inheritance confirmed in 16/21, 1 infant had biallelic PKD2 variants, and 2 infants had digenic PKD1/PKD2 variants. There was no known family history of ADPKD in 13 families (43%) and a de novo pathogenic variant was confirmed in 6 families (23%).
Conclusion
We report a high prevalence of hypomorphic PKD1 variants and likely biallelic disease in infants presenting with PKD-VEO with major implications for reproductive counseling. The diagnostic interpretation and reporting of these variants however remains challenging using current American College of Medical Genetics and Genomics/Association for Molecular Pathology (ACMG/AMP) and Association of Clinical Genetic Science (ACGS) variant classification guidelines in PKD-VEO and other diseases affected by similar variants with incomplete penetrance.
Similar content being viewed by others
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the most common genetic cause of kidney failure and usually presents in adult life.1 Very rarely, ADPKD can be diagnosed in utero or in infancy (up to the age of 18 months) with an often severe, very early onset (VEO) presentation associated with a reported high recurrence rate in subsequent pregnancies.2
The genetic mechanism underlying a VEO presentation has been shown to be related to reduced gene dosage3 with biallelic PKD1 variants being the most consistent finding in several clinical case reports.4,5,6,7 In one study, digenic variants of PKD1 were reported with PKHD1 or HNF1B although these findings have not been confirmed in later studies.8 For instance, a French case series of prenatal onset patients in 41 families with known ADPKD reported 15 biallelic PKD1 variants but did not detect any trans-heterozygous variants in PKD2, PKHD1, and HNF1B.9
To determine the prevalence of these changes in an unselected cohort, we reviewed the results of all infants with a VEO presentation of polycystic kidney disease referred for diagnostic testing to a nationally accredited service laboratory in the UK over a 10-year period. Our results confirm a high prevalence of biallelic PKD1 variants in VEO patients with an enrichment of hypomorphic or reduced penetrance variants, often in heterozygosity with a pathogenic variant. In addition, we identified three cases of biallelic PKD2 variants or trans-heterozygous PKD1 and PKD2 variants. Importantly, a positive family history of ADPKD was only present in 57% of families with a high rate of de novo variants in 23% of families with available parental samples. Although these results have major implications for reproductive counseling, the diagnostic interpretation and reporting of these variants remains challenging using current American College of Medical Genetics and Genomics/Association for Molecular Pathology (ACMG/AMP) and Association of Clinical Genetic Science (ACGS) variant classification guidelines.
MATERIALS AND METHODS
Study population
A retrospective review of ADPKD genetic testing referrals from 2010 to 2020 was performed. All patients with a recorded age of onset of cystic kidneys from prenatal up to 18 months were selected from a large cohort of patients referred to the Sheffield Diagnostic Genetics Service (SDGS) for diagnostic testing. Patients were selected by the date of birth at the time of referral and the specified age of onset of symptoms. Fifty-one patients presenting below 18 months of age were identified. Patients with a clinical history not suggestive of ADPKD or with an alternative genetic diagnosis were subsequently excluded.
Methodology
The methodology used and genes analyzed for each infant are summarized in Table S1. Between 2010 and 2016, genetic testing was carried out by bidirectional Sanger sequencing of all coding exons and ±25 bp intron/exon boundaries of the PKD1 and PKD2 genes. For the duplicated region of PKD1 (exons 1–33), PKD1-specific long-range polymerase chain reaction (PCR) was performed followed by nested PCR and bidirectional sequencing to avoid the six highly homologous PKD1 pseudogenes (primer sequences in Table S2 and on request). Sequencing was analyzed using Mutation Surveyor® software with two independent checks and included a check for pseudogene contamination.
From 2016 to 2018, long-range PCR and next-generation sequencing (NGS) of PKD1 and PKD2 using the IonTorrent Personal Genome Machine (PGM®) was performed. Long-range amplicons were pooled, purified, and enzymatically fragmented (Ion Shear™ Plus), followed by end repair and adapter ligation (Ion Plus Fragment Library Kit). Libraries were sequenced on 316 chip with a minimum depth of coverage of 50× for exons ±5 bp and 30× for introns between ±6 and 25 bp. In-house bioinformatics analysis was performed using the BAM and a VCF file generated by the IonTorrent software. The pipeline used sambamba to determine coverage and ANNOVAR to obtain HGVS nomenclature of variants and their consequences. Only effects on build hg19 transcripts NM_000297 and NM_001009944 were reported with all pseudogene bases masked as N. The results were filtered to remove known benign variants from an in-house manually curated polymorphism list.
From 2018, testing evolved to the use of NGS on HiSeq 2000 with a minimum depth of coverage of 30× for exons ±5 bp and 18× for introns between ±6-25 bp. The designated cystic diagnostic panel comprised 17 genes associated with PKD, polycystic liver disease, and autosomal dominant tubulointerstitial kidney disease: PKD1, PKD2, DNAJB11, GANAB, PKHD1, DZIP1L, PRKCSH, SEC63, SEC61B, SEC61A1, LRP5, ALG8, UMOD, HNF1β, REN, TSC1, and TSC2. Library prep was performed using SureSelectXT library system (Agilent Technologies) and custom in-house designed probes. To aid read alignment to PKD1, the entire sequence of exons 1–33 including all intronic sequences was included in the SureSelect bait capture. Sequencing on the Illumina HiSeq using the HiSeq Rapid SBS Kit v2 performing 2 × 108 bp paired end reads. Bioinformatics analysis based on the open source Best Practices workflow by the Broad Institute, which includes BWA alignment of reads to human genome build hg19, identification of variants using HaplotypeCaller and annotation from dbSNP. Variants were subsequently filtered against in-house benign polymorphism list.
Two infants (cases 4 and 28) were tested using all three methods of long-range PCR and Sanger sequencing, long-range PCR with NGS on PGM®, and SureSelect capture with NGS on Illumina HiSeq 2000. Two infants (cases 22 and 25) were tested by both Sanger sequencing and SureSelect NGS. The results were fully concordant.
One family (case 7) underwent trio exome sequencing using an inheritance-based, gene-agnostic approach. Another family (case 14) underwent trio genome sequencing via the UK 100,000 Genomes Project. Confirmation of the genotypes for cases 7 and 14 and their parents was performed in our laboratory by long-range PCR and Sanger sequencing.
Variant interpretation
All sequence variants identified were assessed and scored according to ACMG/AMP10 and the ACGS best practice guidelines for the evaluation of pathogenicity and the reporting of sequence variants in clinical molecular genetics. Evaluation of pathogenicity included the use of Alamut® Visual 2.11 software, interrogation of available data from PKD variant database (https://pkdb.mayo.edu), Human Gene Mutation Database (HGMD) Professional (https://portal.biobase-international.com/hgmd/pro), and the Genome Aggregation Database (gnomAD) for large exome and genome sequencing studies (https://gnomAD.broadinstitute.org). Predicted evolutionary conservation in silico pathogenicity scores of missense variants were evaluated using REVEL11 (a meta-tool incorporating 13 evolutionary conservation in silico tools) using a cutoff of >0.5 for likely pathogenicity (Table S1). Variants with an ACMG variant classification score of classes 3, 4, or 5 were confirmed by independent Sanger sequencing including the use of long-range PCR for PKD1 exons 1–33. Dosage analysis was performed using MRC-Holland multiplex ligation-dependent probe amplification (MLPA) kits P351 and P352. Variants with a classification of classes 3–5 were reported to the clinician. For familial testing of known variants, two alternative primer sets were used for each amplicon to reduce the possibility of nonamplification of one allele due to single-nucleotide variants (SNVs) under the primer sites.
Molecular modeling of PKD1 variants
PKD1 (6A70) 3D structures were modeled by SWISS-MODEL12 and PHYRE213 automated protein homology modeling server.14 Because no experimental mutant PKD1 structures have been determined, we generated mutant structures by introducing individual missense variants in silico: missense variants were computationally modeled in UCSF Chimera 1.1415 by first swapping amino acids using optimal configurations in the Dunbrack rotamer library16 and by taking into account the most probable rotameric conformation of the mutant residue. All kinds of direct interactions—polar and nonpolar, favorable and unfavorable, including clashes—were analyzed using contacts command in UCSF Chimera 1.14.15 In the output, the atom–atom contacts are listed in order of decreasing van der Waals (VDW) overlap: positive where the atomic VDW spheres are intersecting, zero if just touching, negative if separated by space. The evolutionary conservation score of each amino acid of PKD1 (6A70) was determined using the ConSurf algorithm, based on the phylogenetic relationships between sequence homologs.17,18 The structural impact of missense variants was also analyzed using Missense3D and VarSite.19,20 Lollipop plots were generated using the lollipop variant diagram generator.21
RESULTS
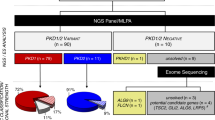
Between 2010 and 2020, a total of 1371 referrals were received for diagnostic PKD genetic testing. The majority were from the UK, with 256 patients (18.7%) referred from 17 other countries. From this cohort, we identified 51 infants with clinical onset before 18 months of age (Fig. 1). Fifteen infants had an alternate genetic diagnosis confirmed, most commonly biallelic PKHD1 pathogenic variants causing ARPKD (9 infants) or an HNF1B deletion (6 infants). In 6 patients, no pathogenic variants were detected in PKD1 or PKD2 but no further testing of additional cystogenes was requested and no confirmation of the original clinical diagnosis could be obtained.
A total of 53 variants were found in the 30/36 infants, of which 47 were unique (43 PKD1 and 4 PKD2) and 14 had not been previously published (Table S1). Sixteen of 30 of these infants presented in utero with cysts and/or enlarged echogenic kidneys visible on antenatal ultrasound scans (Table 1). There was no known family history of ADPKD in 13 (43%) families. In 6 of 26 (23%) families with available parental samples, we were able to confirm a de novo pathogenic variant. Twenty-one infants had two putative variants. In 16 infants (73%) where parental samples were available, we confirmed biallelic in trans inheritance including 2 with de novo variants where phase was established by linkage with nearby variants (case 21) or NGS reads (case 12) (Fig. S1). In three families, a de novo variant of unknown phase was detected. No parental samples were available for testing in the remaining two families.
Biallelic variants (pathogenic and hypomorphic combination)
Sixteen infants had a pathogenic variant on one allele and a missense likely hypomorphic variant on the other allele. The pathogenic variant was inherited from an affected parent in seven cases, from a parent with a positive family history in two cases, from a parent with no cysts and no family history in one case, and was confirmed de novo in four cases. Parental samples were not available to confirm phase for two infants (cases 15 and 16). Inheritance of the likely hypomorphic variant was from an unaffected parent in all cases with parental samples available (27 parents with 0 cysts; 1 parent with 2 renal cysts; 2 families with unknown phase).
Biallelic variants (two hypomorphic alleles)
Five infants had two likely hypomorphic biallelic variants detected in trans (Figs. 1, S1). Two cases (cases 18 and 19) were consanguineous and were homozygous for the likely variant. The variant p.(Ser3037Leu) is novel but p.(Asn3188Ser) has been previously reported in a different consanguineous family4 and two other p.Asn3188 variants, p.(Asn3188Asp) and p.(Asn3188Ile), were reported in the French VEO-PKD cohort.9 Three other cases were compound heterozygous for two likely hypomorphic variants. One parent (case 18) had three unilateral renal cysts (aged 26) while the remaining nine parents had normal renal ultrasounds. Of interest, we detected three PKD1 variants in one infant (case 13) and biallelic PKD2 variants in another (case 5).
Digenic variants
Digenic PKD1 and PKD2 variants were found in two infants (cases 8 and 16). Case 8 has biallelic variants in PKD2 as well as a pathogenic variant in PKD1. Interestingly, the c.11713-2A>T PKD1 pathogenic variant and the p.(Leu736_Asn737del) PKD2 likely pathogenic variant were both inherited from the affected mother, who was diagnosed incidentally in her 20s. However, the fetus also inherited the p.(Val909Ile) PKD2 missense variant from the unaffected father. No additional cases with digenic inheritance of PKD1 or PKD2 variants and PKHD1 or HNF1B were detected in the 20 infants tested for additional cystogenes.
Monoallelic variants (genetically unresolved)
Nine infants had a single PKD1 variant detected, five with cysts detected prenatally (Fig. 1). Five of nine infants had one pathogenic variant detected: three infants inherited a pathogenic variant from an affected parent, one infant had a de novo pathogenic variant, and one pathogenic variant was detected in an infant with a family history of ADPKD but no parental samples available. The remaining four infants had one likely hypomorphic variant inherited from the unaffected parent, concordant with the family history. In two of these families, there was a family history of ADPKD but the causative pathogenic variant in the affected parent had not been detected, consistent with pickup rate of approximately 90% in adult-onset cohorts.9,22 Of the nine infants with a single variant, five underwent further testing on an extended cystic panel. Consent for further analysis was not available for the remaining four patients who had testing limited to PKD1 and PKD2 (three cases) but also included PKHD1 in one case.
In silico analysis
Although the majority of hypomorphic variants detected were unique, five variants were recurrent, either in our study (Table 2) or previously reported. We performed in silico modeling of these recurrent variants using a recent cryoEM structure of truncated (aa3049-4169) human PC1 complexed to PC214 (Fig. 2), complementing this with nuclear magnetic resonance (NMR) modeling of PLAT binding to several putative ligands including Ca2+, phosphatidylserine (PS), phosphatidylinositol-4-phosphate (PI4P), and β-arrestin23 (Fig. 3): p.(Arg3277Cys) cases 1, 2, 28, and refs. 4,5,9; p.(Arg3892His) cases 16 and 17; p.(Ile3167Phe) cases 10, 11, and ref. 24; p.(Asn3188Ser) case 19 and ref. 4; and p.(Glu4025Gly) case 15 and ref. 9
a Lollipop plot showing missense variants relative to a schematic representation of the PC1 protein. Any position with a variant is indicated by a red circle, the gray bar represents the protein with the different amino acid positions (aa), and colored boxes are specific functional domains. b Ribbon diagram of PC1 showing the representative missense variant residues in red. Dashed boxes correspond local impacts of variants on PC1 structure.14 Interactions are labeled as black dashed lines (pseudobonds), H-bonds are labeled in purple and Interatomic distances are expressed in Å.
a Membrane-facing side showing phosphatidylserine, PS (stick), Ca2+ (orange sphere). Surface representation indicating residues binding PS (light blue) and β-arrestin1/2 (pink). The key Ser3164 residue (red) which modulates PI4P and β-arrestin1/2 binding is indicated.23 b Cytoplasmic-facing surface of PLAT showing a predicted protein interaction domain (deep blue). Several residues identified to be altered in this study are labeled (orange) and mapped onto this model, i.e., E3121, I3167, G3150, and N3188. Note the close proximity of E3121 and 13167 to the same Ca2+-dependent PS-binding pocket. E3121 also forms part of a functional YEIL3123 motif involved in AP2-mediated internalization of PC1.23.
Of note, the first two variants lie within the signature PLAT domain (Ile3167, Asn3188). A third residue (Arg3277), lies adjacent to a unique altered residue (Arg3269), both located in the first intracellular loop linking PLAT and the second transmembrane domain (TM2). A fourth residue (Arg3892) lies in the linker between the TOP domain and the TM S2 helix, in close proximity to a nonrecurrent change (Ala3959) in the TM S3 helix. The fifth residue (Glu4025) lies in the TM S5 helix (Fig. 2).
Three of five variants were predicted to significantly alter the structure of the affected domain, i.e., p.(Ile3167Phe), p.(Arg3277Cys), p.(Glu4025Gly) while the other two variants ie p.(Asn3188Ser) and p.(Arg3892His) were predicted to cause more subtle changes (Table S3). It was interesting to note that p.(Arg3892His) was inherited in trans with a second nonrecurrent variant p.(Arg3959Val) in one patient (case 17) as they are in close proximity based on structural modeling despite being in different domains: the combination could have had a more profound effect in altering protein function (Fig. 2).
Missense3D analysis predicted that the p.(Ile3167Phe) substitution results in an altered surface cavity in the PLAT domain and could potentially affect a PS-binding pocket assigned in a previous NMR study that is important for membrane association (Fig. 3a).23 We noted that another altered residue Glu3121 (case 1) lies adjacent to Ile3167, forming part of the same membrane interaction domain. Glu3121 is also part of the functional YEIL3123 AP2-binding motif shown to mediate PC1 internalization.23 Similarly, Gly3150 is localized in a protein interaction domain previously assigned by NMR23 (Fig. 3b). Asn3188 was not predicted to affect known ligand-binding domains but could have a damaging effect on structure (Table S3).
DISCUSSION
In this retrospective analysis of 30 infants with PKD-VEO referred over the past decade, we detected a high prevalence (70%) of biallelic variants, in particular PKD1 hypomorphic variants. These findings have important implications for reproductive genetic counseling since there is a 25% recurrence risk in subsequent pregnancies if both parents are heterozygous for each variant. Around 40% of the infants in our cohort had no previous known family history of ADPKD so the occurrence of a severely affected infant will be highly traumatic for clinically unaffected parents. In this context, we found a high incidence of de novo pathogenic variants (23%) as well as five infants with two hypomorphic variants in trans (17%) causing PKD-VEO. Our results confirm those from a French prenatal cohort, almost all with a positive family history, subjected to more limited genetic analysis (PKD1, PKD2, HNF1B, PKHD1).9 Our detection rate, however, revealed twice the prevalence of biallelic PKD1 variants (70% vs. 37%) and a fivefold higher incidence of de novo PKD1 variants (23% vs. 5%), reflecting the lower percentage of those with a positive family history in our cohort (60% vs. 95%). In contrast to a previous case series describing eight pedigrees,8 we did not detect any infants trans-heterozygous for PKD1 and pathogenic variants in HNF1B or PKHD1 despite more extensive genetic testing in 20 infants (Fig. 1). Other novel findings in our study were three cases of PKD-VEO due to biallelic PKD2 variants and/or trans-heterozygosity for PKD1 and PKD2.
Dosage effect and variability of phenotype severity
There is an emerging consensus that cyst formation in ADPKD arises primarily through a dosage-dependent mechanism centered around PKD1 expression.25 In typical adult-onset disease, patients with PKD1 truncating pathogenic variants develop end-stage renal disease (ESRD) 15 years earlier than those with nontruncating pathogenic variants.26,27 Secondly, informative case reports of PKD-VEO infants with biallelic inheritance of missense hypomorphic PKD1 variants in homozygosity (consanguineous) or heterozygosity with a pathogenic PKD1 truncating variant (in trans) demonstrate the importance of gene dosage in determining phenotypic severity.4,5,9 As observed in mouse studies,3 biallelic complete loss-of-function variants would be incompatible with live births and typically result in a high miscarriage rate or prenatal demise. Therefore, any likely hypomorphic variants detected must retain partial or reduced protein function. The high frequency of missense or in-frame deletions detected in this study and in previous papers are in keeping with this conclusion. Thirdly, patients with pathogenic variants in two new ADPKD genes, GANAB and DNAJB11, have late-onset disease associated with lower but not absent PKD1 expression.28,29 Our findings of biallelic variants in PKD1 and PKD2 are consistent with a dosage effect and their likely function in a polycystin-1/polycystin-2 protein complex and common cystogenic pathway.30 Among our cohort of VEO cases, there was a range of phenotypic severity from severe, including five infants with neonatal demise or TOP, to prenatal onset of cysts with no reported enlargement of kidneys or hypertension. A limitation of our study is that clinical follow-up for all cases was not possible; therefore we cannot exclude a prenatal or neonatal diagnosis of ADPKD due to ascertainment bias in cases with a family history of ADPKD (cases 3, 16, 25, and 27).
Recurrent PKD1 hypomorphic variants
We detected the PKD1 p.(Arg3277Cys) variant in three cases of PKD-VEO, being found in trans in two infants with a second hypomorphic PKD1 allele. In total, this variant has now been reported in six cases of PKD-VEO making it the most common recurrent PKD1 hypomorphic variant associated with this phenotype.4,5,9 We also detected the variants, PKD1 p.(Arg3892His) and p.(Ile3167Phe), each in two cases of PKD-VEO. The p.(Ile3167Phe) variant has been reported in another family with two VEO cases.24 Although previously reported as a likely pathogenic variant,4 it is frequent in population studies, has been found with a truncating pathogenic variant in several pedigrees (phase not established) and listed on the PKD variant database as indeterminate, consistent with a hypomorphic role. p.(Arg3892His) has not been reported previously in association with PKD-VEO, although it has been detected in typical adult-onset patients.9,31 Although rarely detected in population studies, it is still present at a higher frequency than expected for fully penetrant PKD1 alleles. These variants illustrate the difficulty in assigning pathogenicity to potential hypomorphic variants based solely on population frequency.
The high prevalence of hypomorphic PKD1 variants in this context presents a challenge to their interpretation. Currently, we can only infer the likely pathogenicity of the majority of hypomorphic variants from their predicted in silico effects on protein structure or function, evolutionary conservation of the altered residue, previous reported associations, and low frequency in population databases. To confirm a true hypomorphic effect on the PKD1 protein, polycystin-1, functional studies will be required for individual variants. However, there is currently no validated assay that is sufficiently sensitive, robust, and readily accessible. In addition, the size and complexity of polycystin-1 makes this challenging to establish.32 The variant p.(Arg3277Cys) is the only PKD1 hypomorphic variant so far with unequivocal proven reduced function in a genetically engineered mouse mutant (Pkd1 RC mouse).3 Cellular studies have shown that p.(Arg3277Cys) leads to polycystin-1 misfolding, resulting in increased endoplasmic reticulum (ER) retention and reduced surface expression as has been shown for a few other PKD1 missense variants.23 Our use of structural modeling enabled some variants (although not all) to be further refined by their predicted effects on structure and function based on more recently available 3D structures of polycystin-1 and of PLAT. Not all domains currently have experimental structural information but as more structures of polycystin-1 become available, this approach could be applied more systematically.19
Terminology, ACMG classification, and reporting
Hypomorphic variants also cause difficulty with current terminology, ACMG classification and reporting as they do not function as classic loss-of-function variants causing autosomal dominant disease. The terminology is confusing due to the variable language used, which ranges from “hypomorphic,”4 “reduced penetrance”, or “ultralow penetrant”.33 The ClinGen Consortium Low Penetrance/Risk Allele Working Group has recently published guidelines on recommended terminology. For reduced penetrance variants, the use of the ACMG classes plus a quantitative descriptor, i.e., “likely pathogenic, low penetrance” or “likely pathogenic, reduced penetrance,” is recommended depending on sufficient quantitative penetrance estimates. These terms may be used for autosomal dominant disorders where the majority of heterozygous individuals do not develop features of the disease. However, the scenario for PKD1 hypomorphic variants that cause cysts only when inherited in trans with another pathogenic or hypomorphic variant does not fall under this definition. We have therefore continued to use the term “hypomorphic” throughout this publication classifying them by ACMG/ACGS guidelines but labeling the variants with ACMG class, adding H for likely hypomorphic allele.
Since hypomorphic alleles generally have no clinical phenotype in heterozygosity, they may be present in the general population at higher than expected frequencies. For example, the most common hypomorphic p.(Arg3277Cys) variant has been detected on 44 alleles in gnomAD (highest minor allele frequency [MAF] 0.0005 or 0.05%). The other likely hypomorphic variants detected in our cohort had a lower incidence than 44 alleles in gnomAD with two exceptions. p.(Thr2873Ile) is present on 57 alleles in gnomAD but was inherited in cis with the p.(Arg2191His) variant, detected in 37 alleles in gnomAD (case 13): the number of alleles with both variants present is not presently available in gnomAD but it is possible that the combination of both in cis is more deleterious. The c.9499A>T p.(Ile3167Phe) variant has been reported both as an indeterminate variant and observed in trans in another family with two cases of PKD-VEO.24 However it has been detected on 340 alleles in gnomAD including two homozygotes (highest MAF 0.002). Nevertheless, structural modeling suggests that it is likely to be hypomorphic.
Detection of a putative hypomorphic variant confirmed in trans with a pathogenic variant is usually considered highly suspicious and use of the ACMG variant guidelines PM3 (in trans) classifier can often add sufficient weighting to shift the classification into class 4 likely pathogenic. However, the latest Clinical Genomic Resource (ClinGen) sequence variant interpretation (SVI) recommendation for PM3 requires that PM2 is applied, i.e., variants are sufficiently rare in large population studies such as gnomAD. The PM2 threshold for an autosomal dominant fully penetrant disease is 0, a situation very rarely applicable to PKD1 hypomorphic variants. However, if the PM2 threshold for PKD1 hypomorphic variants was relaxed to a maximum of 45 alleles to align with p.(Arg3277Cys) frequency, the PM3 classifier could be used more frequently. Thus the majority of putative hypomorphic variants can only be classified presently as variants of uncertain clinical significance using ACMG. The difficulties in reporting these variants is clearly exemplified by the conflicting classification of the p.(Arg3277Cys) variant on both ClinVar (reported as class 2 likely benign (1), class 4 likely pathogenic (1), and class 5 pathogenic (1) [https://www.ncbi.nlm.nih.gov/clinvar/variation/192320/] and the Human Gene Mutation Database (HGMD) [https://portal.biobase-international.com/hgmd/pro/all.php] where it is listed with conflicting support for pathogenicity.
Hypomorphic or reduced penetrant variants are not unique to PKD1. Many examples are being reported in other diseases such as maturity-onset diabetes of the young (MODY) (e.g., HNF1A,34 RFX6,35 HNF4A),36 Parkinson disease (VPS35,37 LRRK238), retinal dystrophy (ABCA439), and Joubert syndrome (SUFU40). Further clarification is therefore urgently needed to aid variant interpretation and reporting. As the price of NGS has fallen dramatically and many laboratories are now using this technology, it is imperative that classic disease information sources are updated to reflect the prevalence of hypomorphic variants and particularly the alternate inheritance pattern for PKD1 and more rarely PKD2.
Data availability
All methods and data including primer sequences, PCR conditions, software settings, etc. are available on request.
References
Ong AC, Devuyst O, Knebelmann B, Walz G, ERA-EDTA Working Group for Inherited Kidney Diseases. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet. 2015;385:1993–2002.
Zerres K, Rudnik-Schoneborn S, Deget F. Childhood onset autosomal dominant polycystic kidney disease in sibs: clinical picture and recurrence risk. German Working Group on Paediatric Nephrology (Arbeitsgemeinschaft für Pädiatrische Nephrologie). J Med Genet. 1993;30:583–588.
Hopp K, Ward CJ, Hommerding CJ, et al. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest. 2012;122:4257–4273.
Rossetti S, Kubly VJ, Consugar MB, et al. Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int. 2009;75:848–855.
Vujic M, Heyer CM, Ars E, et al. Incompletely penetrant PKD1 alleles mimic the renal manifestations of ARPKD. J Am Soc Nephrol. 2010;21:1097–1102.
Losekoot M, Ruivenkamp CA, Tholens AP, et al. Neonatal onset autosomal dominant polycystic kidney disease (ADPKD) in a patient homozygous for a PKD2 missense mutation due to uniparental disomy. J Med Genet. 2012;49:37–40.
Ali H, Hussain N, Naim M, et al. A novel PKD1 variant demonstrates a disease-modifying role in trans with a truncating PKD1 mutation in patients with autosomal dominant polycystic kidney disease. BMC Nephrol. 2015;16:26.
Bergmann C, von Bothmer J, Ortiz Bruchle N, et al. Mutations in multiple PKD genes may explain early and severe polycystic kidney disease. J Am Soc Nephrol. 2011;22:2047–2056.
Audrezet MP, Corbiere C, Lebbah S, et al. Comprehensive PKD1 and PKD2 mutation analysis in prenatal autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27:722–729.
Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424.
Ioannidis NM, Rothstein JH, Pejaver V, et al. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am J Hum Genet. 2016;99:877–885.
Waterhouse A, Bertoni M, Bienert S, et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303.
Kelley LA, Mezulis S, Yates CM, et al. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858.
Su Q, Hu F, Ge X, et al. Structure of the human PKD1-PKD2 complex. Science. 2018;361:eaat9819.
Pettersen EF, Goddard TD, Huang CC, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612.
Shapovalov MV, Dunbrack RL Jr. A smoothed backbone-dependent rotamer library for proteins derived from adaptive kernel density estimates and regressions. Structure. 2011;19:844–858.
Ashkenazy H, Abadi S, Martz E, et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016;44:W344–W350.
Landau M, Mayrose I, Rosenberg Y, et al. ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 2005;33:W299–W302.
Ittisoponpisan S, Islam SA, Khanna T, et al. Can predicted protein 3D structures provide reliable insights into whether missense variants are disease associated? J Mol Biol. 2019;431:2197–2212.
Laskowski RA, Stephenson JD, Sillitoe I, et al. VarSite: disease variants and protein structure. Protein Sci. 2020;29:111–119.
Jay JJ, Brouwer C. Lollipops in the clinic: information dense mutation plots for precision medicine. PLoS ONE. 2016;11:e0160519.
Rossetti S, Consugar MB, Chapman AB, et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2007;18:2143–2160.
Xu Y, Streets AJ, Hounslow AM, et al. The polycystin-1, lipoxygenase, and alpha-toxin domain regulates polycystin-1 trafficking. J Am Soc Nephrol. 2016;27:1159–1173.
Mantovani V, Bin S, Graziano C, et al. Gene panel analysis in a large cohort of patients with autosomal dominant polycystic kidney disease allows the identification of 80 potentially causative novel variants and the characterization of a complex genetic architecture in a subset of families. Front Genet. 2020;11:464.
Ong AC, Harris PC. A polycystin-centric view of cyst formation and disease: the polycystins revisited. Kidney Int. 2015;88:699–710.
Cornec-Le Gall E, Audrezet MP, Chen JM, et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013;24:1006–1013.
Heyer CM, Sundsbak JL, Abebe KZ, et al. Predicted mutation strength of nontruncating PKD1 mutations aids genotype-phenotype correlations in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27:2872–2884.
Porath B, Gainullin VG, Cornec-Le Gall E, et al. Mutations in GANAB, encoding the glucosidase II alpha subunit, cause autosomal-dominant polycystic kidney and liver disease. Am J Hum Genet. 2016;98:1193–1207.
Cornec-Le Gall E, Olson RJ, Besse W, et al. Monoallelic mutations to DNAJB11 cause atypical autosomal-dominant polycystic kidney disease. Am J Hum Genet. 2018;102:832–844.
Ong ACM. Making sense of polycystic kidney disease. Lancet. 2017;389:1780–1782.
Neumann HP, Jilg C, Bacher J, et al. Epidemiology of autosomal-dominant polycystic kidney disease: an in-depth clinical study for south-western Germany. Nephrol Dial Transplant. 2013;28:1472–1487.
Cai Y, Fedeles SV, Dong K, et al. Altered trafficking and stability of polycystins underlie polycystic kidney disease. J Clin Invest. 2014;124:5129–5144.
Cornec-Le Gall E, Torres VE, Harris PC. Genetic complexity of autosomal dominant polycystic kidney and liver diseases. J Am Soc Nephrol. 2018;29:13–23.
Misra S, Hassanali N, Bennett AJ, et al. Homozygous hypomorphic HNF1A alleles are a novel cause of young-onset diabetes and result in sulfonylurea-sensitive diabetes. Diabetes Care. 2020;43:909–912.
Patel KA, Kettunen J, Laakso M, et al. Heterozygous RFX6 protein truncating variants are associated with MODY with reduced penetrance. Nat Commun. 2017;8:888.
Laver TW, Colclough K, Shepherd M, et al. The common p.R114W HNF4A mutation causes a distinct clinical subtype of monogenic diabetes. Diabetes. 2016;65:3212–3217.
Sharma M, Ioannidis JP, Aasly JO, et al. A multi-centre clinico-genetic analysis of the VPS35 gene in Parkinson disease indicates reduced penetrance for disease-associated variants. J Med Genet. 2012;49:721–726.
Sierra M, Gonzalez-Aramburu I, Sanchez-Juan P, et al. High frequency and reduced penetrance of LRRK2 G2019S mutation among Parkinson’s disease patients in Cantabria (Spain). Mov Disord. 2011;26:2343–2346.
Bauwens M, Garanto A, Sangermano R, et al. ABCA4-associated disease as a model for missing heritability in autosomal recessive disorders: novel noncoding splice, cis-regulatory, structural, and recurrent hypomorphic variants. Genet Med. 2019;21:1761–1771.
De Mori R, Romani M, D’Arrigo S, et al. Hypomorphic recessive variants in SUFU impair the sonic Hedgehog pathway and cause Joubert syndrome with cranio-facial and skeletal defects. Am J Hum Genet. 2017;101:552–563.
Acknowledgements
We are grateful to all referring clinicians and families for providing relevant clinical information, especially Rodney Gilbert and Jackie Cook. We thank all the laboratory team at Sheffield Diagnostic Genetics and Mike Williamson for helpful discussion on NMR modeling. J.C. was supported by an Academic Clinical Fellowship from the UK National Institute for Health Research (NIHR).
Author information
Authors and Affiliations
Contributions
Conceptualization: M.D., J.C., A.O.; Data curation: M.D., J.C.; Formal Analysis: M.D., M.V., P.H.; Investigation: M.D., J.C., M.V.; Resources: M.D., M.V., A.O.; Visualization: M.D., M.V., A.O.; Writing – original draft: M.D., J.C., M.V., A.O.; Writing – review & editing: M.D., P.H., A.O.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no conflicts of interest.
Ethics declaration
All families included in this service review consented for diagnostic genetic testing for ADPKD and/or an extended cystic kidney/liver disease panel in the UK National Health Service (NHS). All data has been de-identified.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Durkie, M., Chong, J., Valluru, M.K. et al. Biallelic inheritance of hypomorphic PKD1 variants is highly prevalent in very early onset polycystic kidney disease. Genet Med 23, 689–697 (2021). https://doi.org/10.1038/s41436-020-01026-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-020-01026-4
This article is cited by
-
Expanding ACMG variant classification guidelines into a general framework
Human Genomics (2022)
-
Changing the Outcome of a Pediatric Disease: Part I — Clinical Features of ADPKD
Current Treatment Options in Pediatrics (2022)
-
The wind of change in the management of autosomal dominant polycystic kidney disease in childhood
Pediatric Nephrology (2022)
-
Translational research approaches to study pediatric polycystic kidney disease
Molecular and Cellular Pediatrics (2021)
-
The genetic landscape of polycystic kidney disease in Ireland
European Journal of Human Genetics (2021)