Abstract
Electrocardiograph (ECG) criteria for left ventricular hypertrophy (LVH) are a widely used clinical tool. We recalibrated six ECG criteria for LVH against gold-standard cardiac magnetic resonance (CMR) and assessed the impact of obesity. One hundred and fifty consecutive tertiary hypertension clinic referrals for CMR (1.5 T) were reviewed. Patients with cardiac pathology potentially confounding hypertensive LVH were excluded (n=22). The final sample size was 128 (age: 51.0±15.2 years, 48% male). LVH was defined by CMR. From a 12-lead ECG, Sokolow–Lyon voltage and product, Cornell voltage and product, Gubner–Ungerleidger voltage and Romhilt–Estes score were evaluated, blinded to the CMR. ECG diagnostic performance was calculated. LVH by CMR was present in 37% and obesity in 51%. Obesity significantly reduced ECG sensitivity, because of significant attenuation in mean ECG values for Cornell voltage (22.2±5.7 vs 26.4±9.4 mm, P<0.05), Cornell product (2540±942 vs 3023±1185 mm • ms, P<0.05) and for Gubner–Ungerleider voltage (18.2±7.1 vs 23.3±1.2 mm, P<0.05). Obesity also significantly reduced ECG specificity, because of significantly higher prevalence of LV remodeling (no LVH but increased mass-to-volume ratio) in obese subjects without LVH (36% vs 16%, P<0.05), which correlated with higher mean ECG LVH criteria values. Obesity-specific partition values were generated at fixed 95% specificity; Cornell voltage had highest sensitivity in non-obese (56%) and Sokolow–Lyon product in obese patients (24%). Obesity significantly lowers ECG sensitivity at detecting LVH, by attenuating ECG LVH values, and lowers ECG specificity through changes associated with LV remodeling. Our obesity-specific ECG partition values could improve the diagnostic performance in obese patients with hypertension.
Similar content being viewed by others
Introduction
In patients with arterial hypertension, left ventricular hypertrophy (LVH) is a powerful independent predictor for sudden cardiac death,1 ventricular arrhythmias,2 myocardial ischemia,3 coronary heart disease4 and heart failure,5 as well as ischemic stroke.6 The 2013 European Society of Hypertension/European Society of Cardiology guidelines for the management of arterial hypertension recommend that a 12-lead electrocardiograph (ECG) be performed for all patients with arterial hypertension, partly to detect the presence of LVH.7 The ECG criteria for LVH have previously been validated against M-mode and two-dimensional echocardiography.8, 9, 10, 11 However, cardiac magnetic resonance (CMR) assessment of left ventricular mass (LVM) has been demonstrated to be more accurate and more reproducible than both M-mode and two-dimensional echocardiography.12, 13, 14 Consequently, CMR is the current noninvasive gold-standard investigation for assessing LVM.15 Yet, there is a paucity of studies comparing the diagnostic performance of the ECG at detecting LVH relative to CMR gold-standard. Furthermore, previous CMR studies (i) have not been in the context of hypertension,16 (ii) have been in unselected CMR referrals for all indications17 or (iii) have been in hypertensive subjects but using older turbogradient echo CMR sequences,18 which have been demonstrated to overestimate LV mass by 13 g m−2 in comparison with the more accurate steady-state free precession (SSFP) sequence.19 It is, therefore, apparent that the ECG criteria for LVH in hypertension have potentially been validated, to date, against variable reference standards. In spite of this, the ECG is invariably the first-line diagnostic tool for the assessment for LVH in hypertensive patients, and treatment decisions may be made on its results. Therefore, understanding the diagnostic performance of the ECG at detecting LVH relative to gold-standard is of paramount importance.
Arterial hypertension and obesity commonly coexist. The latter has been associated with increased LVM and increased prevalence of LVH independent of hypertension.20 However, previous echocardiographic studies into the impact of obesity on the diagnostic performance of the ECG at detecting LVH have demonstrated conflicting results.21, 22, 23, 24
Consequently, the aim of our study was to evaluate the Sokolow–Lyon voltage, Sokolow–Lyon product, Cornell voltage, Cornell product, Gubner–Ungerleider voltage and Romhilt–Estes ECG criteria for LVH in a cohort of tertiary hypertensive clinic patients, with high prevalence of LVH, relative to SSFP CMR measurements of LVM. We also aimed to investigate the effect of obesity and LV remodeling on the ECG criteria for LVH and generate new obesity-specific partition values, by recalibrating the criteria against the current noninvasive gold-standard technique to measure LVM.
Materials and methods
Study population
This was a prospective study of consecutive eligible patients with arterial hypertension referred for CMR from a tertiary hypertension clinic serving the South West of England. Study participants underwent CMR as part of their clinical hypertension work-up between January 2011 and December 2014. In our institution, CMR is used as part of the investigation for secondary causes of hypertension and to quantify hypertensive end-organ damage. To assess hypertensive LVH, exclusion criteria consisted of all concomitant cardiac pathology that may confound the hypertrophic response, including moderate–severe valvular heart disease and clinical or CMR evidence of prior myocardial infarction or other cardiomyopathy. In particular, hypertrophic cardiomyopathy was excluded on the basis of clinical and family history, as well as CMR appearance. There were no high-performing athletes in the cohort, mitigating against the presence of physiological hypertrophy among the subjects.
Baseline demographics and clinical characteristics were recorded. Obesity was defined as a body mass index (BMI) >30 kg m−2. Systolic (SBP) and diastolic (DBP) blood pressure values were the mean office blood pressure readings taken at the time of the ECG of repeat readings from both arms, where available, after the patient had been sitting for 5 min with an appropriately sized cuff.
The local research ethics committee confirmed that the study conformed to the governance arrangements for research ethics committees. The study was conducted with the patient's written consent.
Electrocardiograph
A 12-lead standard ECG (scale: 10 mm=1 mV; speed: 25 mm s−1) was acquired in the supine position during quiet respiration and analyzed by a clinician blinded to the CMR data. Subjects with complete bundle branch block were excluded. The six ECG criteria evaluated for LVH (Table 1) were as follows: Sokolow–Lyon voltage,25 Sokolow–Lyon product,10,26 Cornell voltage,8 Cornell voltage product,10,26 Gubner–Ungerleider voltage27 and Romhilt–Estes score.28
CMR protocol and analysis
CMR was performed at 1.5 T (Avanto, Siemens, Erlangen, Germany). The CMR protocol included short-axis SSFP cines with whole LV coverage (8 mm slice thickness, no slice gap, temporal resolution 38.1 ms, echo time 1.07 ms, in-plane pixel size 1.5 × 0.8 mm2) for the assessment of LVM. In addition, late gadolinium myocardial enhancement was routinely performed using an inversion-recovery fast gradient echo sequence and a phase-sensitive inversion-recovery sequence performed in two-phase-encoding directions ~10–15 min after intravenous administration of 0.1 mmol kg−1 gadobutrol (Gadovist; Bayer Pharma AG, Berlin, Germany). Tailored inversion times were used in each patient to achieve myocardial nulling. Subjects with subendocardial late gadolinium myocardial enhancement consistent with previous myocardial infarction were excluded. Routine renovascular assessment was also part of routine hypertension work-up, consisting of Time-resolved angiography With Interleaved Stochastic Trajectories (TWIST) contrast-enhanced magnetic resonance angiography, which creates multiphase, multiplanar images of the abdominal vasculature.
The assessment of LVM was performed as described previously, which also used thresholding software analysis.29 Briefly, endocardial contours were defined at end-diastole and end-systole on the LV short-axis stack using blood pool/endocardial border threshold detection software (cmr42; Circle Cardiovascular Imaging Inc., Calgary, AB, Canada), which has been previously validated.30 Epicardial contours were defined manually at end-diastole. The LVM was estimated by multiplying the total myocardial volume, including papillary muscles and LV trabeculations (equivalent to LV dry weight), by 1.05 g ml−1, which is the specific gravity of myocardium, as described previously.29 The LVM and end-diastolic and end-systolic volumes were indexed to body surface area, calculated using the Mosteller formula. LVH was defined as indexed LVM >95th percentile of established CMR reference ranges indexed to body surface area (men: 89–93 g m−2 and women: 77–78 g m−2 depending on age).29 LV remodeling was defined as a ventricle with normal indexed LVM but elevated LV mass/volume ratio (M/V).16 An increased M/V was defined as >95th gender-specific percentile (men: >1.12 g ml−1 and women: >1.14 g ml−1) from healthy volunteers, as described previously.16 Asymmetric wall thickness was defined as a regional wall thickness ⩾13 mm also >1.5-fold the thickness of the contralateral myocardial segment.31 The CMR analysis was performed by an experienced CMR reader blinded to the ECG data. All CMR readings were repeated twice and the mean value used for analysis. The intraobserver percentage variability in repeat measurements was 3.4±2.5% and the intraobserver intraclass correlation coefficient (two-way mixed, absolute agreement, average measures) was 0.991 (95% confidence interval (CI): 0.987–0.994).
Statistical analysis
Statistical analysis was performed using SPSS version 21 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean±s.d. and normally distributed variables were compared using unpaired Student’s T-tests or one-way analysis of variance with least significant difference post hoc correction as appropriate. Categorical variables were expressed as percentages and analyzed using Fisher’s exact or χ2 tests as appropriate. Sensitivity, specificity, positive predictive valve (PPV), negative predictive value (NPV) and accuracy were also calculated. Receiver-operating characteristics (ROC) analysis was carried out to calculate the area under the curve (AUC) and to compare the diagnostic performance of the various ECG criteria. Statistical significance was set as two-tailed P<0.05.
Results
Demographics
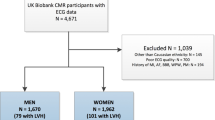
One hundred and fifty consecutive patients referred for CMR were assessed for eligibility. Twenty-two patients met the exclusion criteria (Figure 1), resulting in a final sample size of 128 (men: 49%; age: 51.0±15.2 years) (Table 2). All subjects completed the CMR study. CMR evidence of LVH was present in 37% (n=47) and obesity was present in 51% (n=65) of the cohort. There were no significant differences between non-obese (n=63) and obese subjects (n=65) in age (50.7±16.7 vs 51.3±13.6 years, P=0.803), gender (men: 52% vs 46%, P=0.596), SBP (171.9±30.0 vs 169.6±30.1 mm Hg, P=0.6641), DBP (98.1±16.0 vs 96.9±14.9 mm Hg, P=0.6604) or CMR prevalence of LVH (29% vs 45%, P=0.069). The majority (92%) of patients were taking at least one antihypertensive medication.
A flow chart demonstrating the study exclusion criteria and final sample size (n=128). *Image artifact from implantable loop recorder device precluding volumetric assessment from LV short-axis SSFP cine stack. AVR, aortic valve replacement; DCM, idiopathic dilated cardiomyopathy (CMR diagnosis); ECG, electrocardiogram; HOCM, hypertrophic obstructive cardiomyopathy (clinical and/or CMR diagnosis); LVNC, left ventricular non-compaction cardiomyopathy (CMR diagnosis); MI, myocardial infarction (defined as subendocardial late gadolinium enhancement on CMR); Mod AR, moderate aortic regurgitation.
Indexed LVM in subjects with positive ECG criteria
Using established partition values, the mean indexed LVM in those subjects with ECG evidence of LVH was significantly higher compared in those without ECG evidence of LVH for all the ECG criteria (Sokolow–Lyon voltage: 96.6±28.4 vs 82.7±20.4 g m−2, P<0.05; Sokolow–Lyon product: 99.8±31.2 vs 82.9 ±20.3 g m−2, P<0.05; Cornell voltage: 96.3±28.6 vs 80.2±17.3 g m−2, P<0.0005; Cornell product: 93.1±27.7 vs 79.6±16.2 g m−2, P<0.005; Gubner–Ungerleider voltage: 92.4±24.8 vs 80.5±19.3 g m−2, P<0.005; Romhilt–Estes 4p: 97.0±25.5 vs 76.5±14.6 g m−2, P<0.0001; and Romhilt–Estes 5p: 101.8±31.3 vs 80.9±17.7 g m−2, P<0.0001).
Diagnostic performance of the ECG criteria of LVH
Specificity was higher than sensitivity for all the ECG criteria investigated (Table 3), using the traditional partition values described in Table 1. The Sokolow–Lyon product yielded the highest specificity (96%), and the highest sensitivity (55%) was achieved by the Romhilt–Estes 4p criterion. However, the Cornell voltage criterion performed best on AUC-ROC analysis (0.752, 95th CI: 0.666–0.809). By obesity subgroup analysis (Table 4), the sensitivity for obese subjects compared with non-obese subjects was significantly lower for Cornell voltage (34% vs 56%, P<0.05), Cornell product (48% vs 61%, P<0.05), Gubner–Ungerleider voltage (41% vs 67%, P<0.05) and Romhilt–Estes 4p score (45% vs 72%, P<0.05). Similarly, the specificity was significantly lower for Cornell product (69% vs 83%, P<0.05) and Gubner–Ungerleider voltage (61% vs 82%, P<0.05) in the presence of obesity. The ROC-AUC analysis was inferior for obese subjects relative to non-obese subjects for Cornell voltage, Cornell product and Gubner–Ungerleider voltage criteria.
Obesity subgroup analysis of the ECG criteria for LVH
The mean values for each ECG criterion were calculated for individuals with CMR evidence of LVH and those without CMR evidence of LVH, by obesity subgroup (Table 5). In the presence of LVH by CMR, the mean values for Cornell voltage (26.4±9.4 vs 22.2±5.7 mm, P<0.05), Cornell product (3023±1185 vs 2540±942 mm • ms, P<0.05) and for Gubner–Ungerleider voltage (23.3±1.2 vs 18.2±7.1 mm, P<0.05) were significantly lower for obesity subjects compared with non-obese subjects. Similar, albeit nonsignificant, trends were demonstrated for Sokolow–Lyon voltage, Sokolow–Lyon product and Romhilt–Estes score criteria. This was despite a trend toward higher mean indexed LVM in obese subjects with LVH compared with non-obese subjects with LVH (108.3±21.4 vs 102.0±18.1 kg m−2, P=0.3047).
However, in the absence of LVH by CMR, there was no significant difference between the mean ECG criteria values for subjects with obesity and subjects without obesity (Table 5). In fact, there were nonsignificant trends toward higher mean ECG criteria values in obese individuals without CMR evidence of LVH. Interestingly, the prevalence of LV remodeling (normal indexed LVM but increased M/V) was significantly higher in those with obesity compared with non-obese subjects (36% vs 16%, P<0.05) and consequently the prevalence of structurally normal left ventricles was significantly lower (64% vs 84%, P<0.05). There was a significantly higher mean value for the Cornell voltage for subjects with remodeling compared with those with a structurally normal LV (19.8±5.1 vs 16.0±5.8 mm, P<0.05), in the absence of a significant gender difference between the cohorts. Similar, but nonsignificant, trends were observed for Cornell product (2129±51.4 vs 1987±64 mm • ms, P=0.3717) and Gubner–Ungerleider voltage (16.6±5.4 vs 14.5±7.0 mm, P=0.2235) criteria. These trends corresponded to a significantly increased mean indexed LVM, but still within the normal range, for subjects with remodeling compared with subjects with normal LV structure (78.9±9.1 vs 69.6±8.7 kg m−2, P<0.0001).
Obesity-specific partition values
To facilitate comparison of sensitivities, the ECG criteria sensitivities were calculated relative to a fixed 95% specificity, as described previously.10, 11, 18, 26 The obesity-specific partition values derived from our cohort are demonstrated in Table 6. At a fixed 95% specificity, the Cornell voltage had the highest sensitivity (56%) among non-obese subjects. The recalculated sensitivities were similar for all criteria in the presence of obesity, but the highest was achieved with the Sokolow–Lyon product (24%). Obesity-specific partition values are presented in Table 6.
Discussion
This is the first study to investigate the impact of obesity on the diagnostic performance of the ECG at detecting LVH relative to CMR gold-standard using SSFP sequences in a clinical hypertensive cohort.
We have demonstrated that, in hypertensive subjects with ECG evidence of LVH, indexed LVM is significantly elevated compared with subjects without ECG evidence for LVH. However, all of the ECG criteria were poor at excluding LVH as defined by CMR. Furthermore, the ability of the ECG to exclude LVH deteriorates in the presence of obesity.
Previous echocardiographic studies have demonstrated the impact of obesity on the diagnostic performance of the ECG criteria for echocardiographic defined LVH. Our results demonstrate that sensitivities of praecordial lead ECG voltage criteria for the detection of LVH were reduced in the presence of obesity, which is consistent with a number of such previous studies.21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 We show that obese subjects with CMR-proven LVH have significantly lower mean values for Cornell voltage, Cornell product and Gubner–Ugerleider voltage compared with non-obese subjects. There were similar, albeit nonsignificant, trends toward lower values for the other ECG criteria investigated. On the one hand, previous echocardiographic studies have found no significant differences for the Cornell voltage,23, 34 on the other hand, one study showed an obesity-related increase in the mean values for Cornell voltage and Cornell product.35 In this latter study by Okin et al.,35 the increase has been explained by obesity-associated increase in LVM, and in our cohort, we demonstrated a nonsignificant trend toward higher mean indexed LVM in obese subjects with LVH compared with non-obese subjects with LVH, and despite this, the ECG criteria values were still attenuated. Nevertheless, there is consensus between our results and those of Okin et al.35 that the Cornell product provides a high sensitivity for obese subjects using traditional partition values.
The inferior sensitivity of the ECG at detecting LVH in the presence of obesity has previously been explained by the increased distance of the ECG electrodes from the heart, which may reduce QRS amplitudes.21 Our results provide further insight into the mechanism. If the impact of obesity on the ECG criteria values were simply an insulating phenomenon related to increased subcutaneous adipose tissue, similar trends toward lower ECG criteria values should also be observed in obese individuals without LVH compared with non-obese individuals without LVH. We found no significant difference between these cohorts. It is important to realize that the absence of LVH does not equate to a normal ventricular structure. In those without CMR evidence of LVH, significantly more subjects with obesity had LV remodeling, as defined as no LVH but an abnormally elevated mass:volume ratio suggesting reduction in LV cavity size, compared with non-obese subjects. An equilibrium between increased LVM associated with obesity and the increased electrical insulation of additional adipose tissue likely determines whether the ECG criteria values demonstrate any significant change in obese vs non-obese individuals. In those with LVH in our cohort, the insulating properties would appear to counteract the trend toward increased indexed LVM in the obese vs non-obese subjects. Our results suggest that obesity-related increases in mass:volume ratio result in increased ECG LVH criteria values in subjects without LVH. The impact of LV remodeling appears to offset any expected additional electrical insulation properties in the obese individuals without LVH in our cohort. This may account for the reduction in specificity of the ECG at detecting LVH in obesity. However, the reasons how and why an increase in LVM or M:V dominates over increased electrical insulation in certain obese individuals remains uncertain.
Our findings of significantly reduced ECG sensitivity and specificity at detecting LVH in the presence of obesity suggest that a normal ECG in an obese hypertensive patient has a higher chance of being both falsely reassuring for the absence of LVH and falsely positive in obese compared with non-obese subjects. Failing to identify or exclude LVH may alter an individual’s cardiovascular risk estimate and therefore may have treatment implications.7 Our obesity-specific partition values may help to readdress this issue. At a fixed 95% specificity, sensitivity was inferior for obese subjects compared with non-obese subjects for all criteria except Sokolow–Lyon voltage and Sokolow–Lyon product. Sensitivity and specificity need to be interpreted in the context of the disease prevalence and these results are likely related to the relatively low prevalence of ECG LVH with the Sokolow–Lyon voltage and product criteria (Table 2). The lower prevalence of ECG LVH with these criteria is consistent with previous work from Okin et al.35 The best sensitivity for non-obese subjects (56%) was achieved with the Cornell voltage criterion, which is consistent with a previous study by Molloy et al.,26 who demonstrated a sensitivity of 50% for the Cornell product criterion for antemortem ECG compared with LVM measured by dry weight at autopsy.26 The optimal sensitivity for obese subjects was achieved with the Sokolow–Lyon product, using an amended partition value of ⩾3275 mm • ms compared with the conventional ⩾3710 mV ms−1.
Limitations
It is important to realize that direct comparison between our study and existing echocardiography studies is subject to discrepancy as our analysis accounted for the influences of age as well as gender on LVM by varying the definition of LVH in relation to these variables in accordance with established normal ranges.29 Gender-specific definitions of LVH only were used in the previous echocardiographic studies.8, 9, 10 Furthermore, blood pool thresh-holding software enabled inclusion of papillary muscles and LV trabeculation in the estimation of LVM to yield as close a noninvasive estimate of dry LV weight as possible in our study. Consequently, the thresholds and accuracy for defining LVH will be different between studies, and, as a result, the proportion of individuals classified as having LVH will also differ. Furthermore, differing allometric scaling of LVM between studies will alter the definition of LVH and impact on the diagnostic performance of the ECG.24
In this clinical cohort study, most patients were receiving antihypertensive medications. Patients with myocardial infarction and valvular heart disease were excluded. A further study is therefore required in normotensive individuals and less highly selected hypertensive patients, for example, untreated hypertensive patients, before the results can be widely extrapolated.
Our study size of 128 patients was modest. However, the findings are still likely valid because of the compensation afforded by the increased accuracy and reproducibility of CMR. For example, previous work by Myerson et al.36 has demonstrated that the sample size for CMR can be approximately 6-fold lower than two-dimensional echocardiography and 12-fold lower than M-mode echocardiography for a power (1−β error) of 95% to detect a statistically significant change in mean LV mass of 10 g.
Total body fat mass is not routinely measured in our clinical practice and could not be investigated. BMI was used as a routinely recorded clinical surrogate. An increase in lean muscle mass may generate elevated BMI values and the partition values generated may not be applicable in this context, although a BMI >30 kg m−2 because of increased lean muscle mass is unlikely to occur, except in very high-performing athletes.
Conclusion
ECG evidence of LVH identifies patients with significantly elevated indexed LVM, measured with noninvasive gold-standard SSFP CMR, for all the ECG criteria investigated.
However, lower sensitivity compared with specificity was demonstrated for all the ECG criteria, with further inferior sensitivities for obese subjects for the majority of ECG criteria investigated. Obesity attenuates the ECG criteria values in the presence of LVH. In addition, we have shown that obesity-related LV remodeling may influence the diagnostic performance of the ECG at detecting LVH. The application of obesity-specific partition values may help improve the diagnostic performance of the ECG in this important subgroup of patients with hypertension.

References
Kannel WB, Doyle JT, McNamara PM, Quickenton P, Gordon T . Precursors of sudden coronary death. Factors related to the incidence of sudden death. Circulation 1975; 51 (4): 606–613.
McLenachan JM, Henderson E, Morris KI, Dargie HJ . Ventricular arrhythmias in patients with hypertensive left ventricular hypertrophy. N Engl J Med 1987; 317 (13): 787–792.
Dunn FG, Pringle SD . Left ventricular hypertrophy and myocardial ischemia in systemic hypertension. Am J Cardiol 1987; 60 (17): 19I–22I.
Liao Y, Cooper RS, McGee DL, Mensah GA, Ghali JK . The relative effects of left ventricular hypertrophy, coronary artery disease, and ventricular dysfunction on survival among black adults. JAMA 1995; 273 (20): 1592–1597.
Kannel WB, Castelli WP, McNamara PM, McKee PA, Feinleib M . Role of blood pressure in the development of congestive heart failure. The Framingham study. N Engl J Med 1972; 287 (16): 781–787.
Fox ER, Alnabhan N, Penman AD, Butler KR, Taylor HA, Skelton TN et al. Echocardiographic left ventricular mass index predicts incident stroke in African Americans: Atherosclerosis Risk in Communities (ARIC) Study. Stroke 2007; 38 (10): 2686–2691.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M et al. ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34 (28): 2159–2219.
Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS et al. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol 1985; 6 (3): 572–580.
Schillaci G, Verdecchia P, Borgioni C, Ciucci A, Guerrieri M, Zampi I et al. Improved electrocardiographic diagnosis of left ventricular hypertrophy. Am J Cardiol 1994; 74 (7): 714–719.
Okin PM, Roman MJ, Devereux RB, Kligfield P . Electrocardiographic identification of increased left ventricular mass by simple voltage-duration products. J Am Coll Cardiol 1995; 25 (2): 417–423.
Norman JE, Levy D, Campbell G, Bailey JJ . Improved detection of echocardiographic left ventricular hypertrophy using a new electrocardiographic algorithm. J Am Coll Cardiol 1993; 21 (7): 1680–1686.
Myerson SG, Montgomery HE, World MJ, Pennell DJ . Left ventricular mass: reliability of M-mode and 2-dimensional echocardiographic formulas. Hypertension 2002; 40 (5): 673–678.
Germain P, Roul G, Kastler B, Mossard JM, Bareiss P, Sacrez A . Inter-study variability in left ventricular mass measurement. Comparison between M-mode echography and MRI. Eur Heart J 1992; 13 (8): 1011–1019.
Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS . Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens 1995; 8 (3): 221–228.
Pennell DJ . Ventricular volume and mass by CMR. J Cardiovasc Magn Reson 2002; 4 (4): 507–513.
Buchner S, Debl K, Haimerl J, Djavidani B, Poschenrieder F, Feuerbach S et al. Diagnosis of left ventricular hypertrophy in aortic valve disease. Evaluation of ECG criteria by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2009; 11: 1–11.
Krittayaphong R, Nomsawadi V, Muenkaew M, Miniphan M, Yindeengam A, Udompunturak S . Accuracy of ECG criteria for the diagnosis of left ventricular hypertrophy: a comparison with magnetic resonance imaging. J Med Assoc Thai 2013; 96 (Suppl 2): S124–S132.
Alfakih K, Walters K, Jones T, Ridgway J, Hall AS, Sivananthan M . New gender-specific partition values for ECG criteria of left ventricular hypertrophy: recalibration against cardiac MRI. Hypertension 2004; 44 (2): 175–179.
Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU . Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imag 2003; 17 (3): 323–329.
Lauer MS, Anderson KM, Levy D . Separate and joint influences of obesity and mild hypertension on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol 1992; 19 (1): 130–134.
Horton JD, Sherber HS, Lakatta EG . Distance correction for precordial electrocardiographic voltage in estimating left ventricular mass: an echocardiographic study. Circulation 1977; 55 (3): 509–512.
Devereux RB, Phillips MC, Casale PN, Eisenberg RR, Kligfield P . Geometric determinants of electrocardiographic left ventricular hypertrophy. Circulation 1983; 67 (4): 907–911.
Abergel E, Tase M, Menard J, Chatellier G . Influence of obesity on the diagnostic value of electrocardiographic criteria for detecting left ventricular hypertrophy. Am J Cardiol 1996; 77 (9): 739–744.
Okin PM, Roman MJ, Devereux RB, Kligfield P . Electrocardiographic identification of left ventricular hypertrophy: test performance in relation to definition of hypertrophy and presence of obesity. J Am Coll Cardiol. 1996; 27 (1): 124–131.
Sokolow M, Lyon TP . The ventricular complex in right ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949; 38 (2): 273–294.
Molloy TJ, Okin PM, Devereux RB, Kligfield P . Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol 1992; 20 (5): 1180–1186.
Gubner R, Ungerleider H . Electrocardiographic criteria of left ventricular hypertrophy. Arch Intern Med 1943; 72: 196–209.
Romhilt DW, Estes EH . A point-score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J 1968; 75 (6): 752–758.
Maceira A, Prasad S, Khan M, Pennell D . Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2006; 8 (3): 417–426.
Childs H, Ma L, Ma M, Clarke J, Cocker M, Green J et al. Comparison of long and short axis quantification of left ventricular volume parameters by cardiovascular magnetic resonance, with ex-vivo validation. J Cardiovasc Magn Reson 2011; 13 (1): 40.
Dweck MR, Joshi S, Murigu T, Gulati A, Alpendurada F, Jabbour A et al. Left ventricular remodeling and hypertrophy in patients with aortic stenosis: insights from cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012; 14 (1): 50.
Rodriguez CJ, Diez-Roux AV, Moran A, Jin Z, Kronmal RA, Lima J et al. Left ventricular mass and ventricular remodeling among Hispanic subgroups compared with non-Hispanic blacks and whites: MESA (Multi-ethnic Study of Atherosclerosis). J Am Coll Cardiol 2010; 55 (3): 234–242.
Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP . Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation 1990; 81 (3): 815–820.
Rautaharju PM, Zhou SH, Calhoun HP . Ethnic differences in ECG amplitudes in North American white, black, and Hispanic men and women. Effect of obesity and age. J Electrocardiol 1994; 27 (Suppl): 20–31.
Okin PM, Jern S, Devereux RB, Kjeldsen SE, Dahlof B, Group FTLS . Effect of obesity on electrocardiographic left ventricular hypertrophy in hypertensive patients: The Losartan Intervention For Endpoint (LIFE) Reduction in Hypertension Study. Hypertension 2000; 35 (1): 13–18.
Myerson SG, Bellenger NG, Pennell DJ . Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension 2002; 39 (3): 750–755.
Acknowledgements
This work was supported by NIHR Cardiovascular Biomedical Research Unit, Bristol Heart Institute. We thank Mr Christopher Lawton, CMR Superintendent Radiographer, and his team of CMR Radiographers in the Bristol Heart Institute for their expertise in acquiring the CMR studies. The views expressed are those of the authors and not necessarily those of the National Health Service, National Institute for Health Research or Department of Health. JCLR is funded by a Clinical Society of Bath Postgraduate Research Bursary 2014 and the Royal College of Radiologists Kodak Research Scholarship 2014. ECH is funded by BHF Grant IBSRF FS/11/1/28400.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Bucciarelli-Ducci is a Consultant for Circle Cardiovascular Imaging Inc., Calgary, AB, Canada.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Rodrigues, J., McIntyre, B., Dastidar, A. et al. The effect of obesity on electrocardiographic detection of hypertensive left ventricular hypertrophy: recalibration against cardiac magnetic resonance. J Hum Hypertens 30, 197–203 (2016). https://doi.org/10.1038/jhh.2015.58
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2015.58
This article is cited by
-
Patterns and associated factors of electrocardiographic abnormality among type 2 diabetic patients in Amhara National Regional State Referral Hospitals, Ethiopia: a multicenter institution-based cross-sectional study
BMC Cardiovascular Disorders (2022)
-
Obesity Potentiates the Risk of Drug-Induced Long QT Syndrome - Preliminary Evidence from WNIN/Ob Spontaneously Obese Rat
Cardiovascular Toxicology (2021)
-
Electrocardiographic detection of hypertensive left atrial enlargement in the presence of obesity: re-calibration against cardiac magnetic resonance
Journal of Human Hypertension (2017)