Abstract
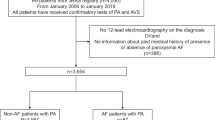
Primary aldosteronism (PA) is the most common endocrine form of hypertension and may carry an increased risk of atrial flutter or fibrillation (AFF). The primary goal of this multicentre cohort study is thus to prospectively establish the prevalence of PA in consecutive hypertensive patients referred for lone (non-valvular), paroxysmal or permanent AFF. Secondary objectives are to determine: (1) the predictors of AFF in patients with PA; (2) the rate of AFF recurrence at follow-up after specific treatment in the patients with PA; (3) the effect of AFF that can increase atrial natriuretic peptide via the atrial stretch and thereby blunt aldosterone secretion, on the aldosterone-to-renin ratio (ARR), and thus the case detection of PA; (4) the diagnostic accuracy of ARR based on plasma renin activity or on the measurement of active renin (DRA) for diagnosing PA in AFF patients. Case detection and subtyping of PA will be performed according to established criteria, including the ‘four corners criteria’ for diagnosing aldosterone-producing adenoma. Pharmacologic or direct current cardioversion will be undertaken whenever indicated following current guidelines. The hormonal values and ARR will be compared within patient between AFF and sinus rhythm. Organ damage, cardiovascular events and recurrence of AFF will also be assessed during follow-up in patients with PA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol 2006; 48: 2293–2300.
Beevers DG, Brown JJ, Ferriss JB, Fraser R, Lever AF, Robertson JI et al. Renal abnormalities and vascular complications in primary hyperaldosteronism. Evidence on tertiary hyperaldosteronism. Q J Med 1976; 45: 401–410.
Rossi G, Boscaro M, Ronconi V, Funder JW . Aldosterone as a cardiovascular risk factor. Trends Endocrinol Metab 2005; 16: 104–107.
Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ . Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 2005; 45: 1243–1248.
Porodko M, Auer J, Eber B . Conn's syndrome and atrial fibrillation. Lancet 2001; 357: 1293–1294.
Watson T, Karthikeyan VJ, Lip GY, Beevers DG . Atrial fibrillation in primary aldosteronism. J Renin Angiotensin Aldosterone Syst 2009; 10: 190–194.
Muiesan ML, Salvetti M, Monteduro C, Donato F, Rizzoni D, Agabiti-Rosei E . Various ways of calculating echocardiographic left ventricular mass and their relative prognostic values. J Hypertens 1998; 16: 1201–1206.
Vaziri SM, Larson MG, Lauer MS, Benjamin EJ, Levy D . Influence of blood pressure on left atrial size. The Framingham Heart Study. Hypertension 1995; 25: 1155–1160.
Rossi GP, Sacchetto A, Pavan E, Palatini P, Graniero GR, Canali C et al. Remodeling of the left ventricle in primary aldosteronism due to Conn's adenoma. Circulation 1997; 95: 1471–1478.
Sun Y, Weber KT . Cardiac remodelling by fibrous tissue: role of local factors and circulating hormones. Ann Med 1998; 30 (Suppl 1): 3–8.
Ramires FJ, Sun Y, Weber KT . Myocardial fibrosis associated with aldosterone or angiotensin II administration: attenuation by calcium channel blockade. J Mol Cell Cardiol 1998; 30: 475–483.
Rossi GP, Di Bello V, Ganzaroli C, Sacchetto A, Cesari M, Bertini A et al. Excess aldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension 2002; 40: 23–27.
Franklin SS, Wachtell K, Papademetriou V, Olsen MH, Devereux RB, Fyhrquist F et al. Cardiovascular morbidity and mortality in hypertensive patients with lower versus higher risk: a LIFE substudy. Hypertension 2005; 46: 492–499.
Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359: 995–1003.
Schmieder RE, Kjeldsen SE, Julius S, McInnes GT, Zanchetti A, Hua TA et al. Reduced incidence of new-onset atrial fibrillation with angiotensin II receptor blockade: the VALUE trial. J Hypertens 2008; 26: 403–411.
Healey JS, Baranchuk A, Crystal E, Morillo CA, Garfinkle M, Yusuf S et al. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol 2005; 45: 1832–1839.
Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE . Prevention of atrial fibrillation by Renin-Angiotensin system inhibition a meta-analysis. J Am Coll Cardiol 2010; 55: 2299–2307.
Rossi GP . Cardiac consequences of aldosterone excess in human hypertension. Am J Hypertens 2006; 19: 10–12.
Thrall G, Lane D, Carroll D, Lip GY . Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006; 119: 448.e1–448.e19.
Sukor N, Kogovsek C, Gordon RD, Robson D, Stowasser M . Improved quality of life, blood pressure, and biochemical status following laparoscopic adrenalectomy for unilateral primary aldosteronism. J Clin Endocrinol Metab 2010; 95: 1360–1364.
European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery, Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31: 2369–2429.
Alhadramy O, Jeerakathil TJ, Majumdar SR, Najjar E, Choy J, Saqqur M . Prevalence and predictors of paroxysmal atrial fibrillation on Holter monitor in patients with stroke or transient ischemic attack. Stroke 2010; 41: 2596–2600.
Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011; 123: 1501–1508.
Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008; 93: 3266–3281.
Rossi GP . Medscape. A comprehensive review of the clinical aspects of primary aldosteronism. Nat Rev Endocrinol 2011; 7: 485–495.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18: 1440–1463.
Leung DY, Chi C, Allman C, Boyd A, Ng AC, Kadappu KK et al. Prognostic implications of left atrial volume index in patients in sinus rhythm. Am J Cardiol 2010; 105: 1635–1639.
Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med 2006; 354: 934–941.
Marchese P, Bursi F, Delle Donne G, Malavasi V, Casali E, Barbieri A et al. Indexed left atrial volume predicts the recurrence of non-valvular atrial fibrillation after successful cardioversion. Eur J Echocardiogr 2011; 12: 214–221.
Rossi GP, Pessina AC, Heagerty AM . Primary aldosteronism: an update on screening, diagnosis and treatment. J Hypertens 2008; 26: 613–621.
Rossi GP, Barisa M, Belfiore A, Desideri G, Ferri C, Letizia C et al. The aldosterone-renin ratio based on the plasma renin activity and the direct renin assay for diagnosing aldosterone-producing adenoma. J Hypertens 2010; 28: 1892–1899.
Campbell DJ, Nussberger J, Stowasser M, Danser AH, Morganti A, Frandsen E et al. Activity assays and immunoassays for plasma Renin and prorenin: information provided and precautions necessary for accurate measurement. Clin Chem 2009; 55: 867–877.
Rossi GP, Seccia TM, Palumbo G, Belfiore A, Bernini G, Caridi G et al. Within-patient reproducibility of the aldosterone: renin ratio in primary aldosteronism. Hypertension 2010; 55: 83–89.
Kageyama Y, Suzuki H, Saruta T . Effects of routine heparin therapy on plasma aldosterone concentration. Acta Endocrinol (Copenh) 1991; 124: 267–270.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470.
Rossi GP, Belfiore A, Bernini G, Desideri G, Fabris B, Ferri C et al. Prospective evaluation of the saline infusion test for excluding primary aldosteronism due to aldosterone-producing adenoma. J Hypertens 2007; 25: 1433–1442.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rossi, G., Seccia, T., Gallina, V. et al. Prospective appraisal of the prevalence of primary aldosteronism in hypertensive patients presenting with atrial flutter or fibrillation (PAPPHY Study): rationale and study design. J Hum Hypertens 27, 158–163 (2013). https://doi.org/10.1038/jhh.2012.21
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2012.21
Keywords
This article is cited by
-
Management of primary aldosteronism and mineralocorticoid receptor-associated hypertension
Hypertension Research (2020)
-
Effect of unilateral adrenalectomy on the quality of life of patients with lateralized primary aldosteronism
BMC Surgery (2019)
-
Arterial Hypertension, Aldosterone, and Atrial Fibrillation
Current Hypertension Reports (2019)
-
Effetti extra-renali dei mineralcorticoidi: non solo sale!
L'Endocrinologo (2017)