Abstract
The role of TPO gene polymorphism in the susceptibility to Graves’ disease (GD) remains unclear. However, single-nucleotide polymorphisms (SNPs) near TPO have been recently associated with serum levels of thyroid peroxidase (TPO) antibody in two independent genome-wide association studies. Moreover, we have observed a strong association between the rs11675434 SNP located near TPO and the presence of clinically evident Graves’ ophthalmopathy (GO). The aim of the current study was to reevaluate and dissect this association in an extended group of 1231 well-characterized patients with GD (1043 adults and 188 children) and 1130 healthy controls from the Polish Caucasian population, considering possible gender-dependent and age-of-onset-specific effects of the studied SNP. We found that the T allele of rs11675434 was significantly more frequent in GD patients with than without GO (odds ratio (OR)=1.26, 95% confidence interval (CI)=1.05–1.51, P=0.012), which was consistent with our previous findings. Further analyses performed in subgroups of patients showed that the association with GO was significant in adult patients with age of GD onset ⩾45 years (OR=1.34, 95% CI=1.03–1.75, P=0.031), but not in children and adolescents or adult patients with earlier onset of the disease (OR=1.72, 95% CI=0.77–3.84, P=0.18 and OR=1.05, 95% CI=0.79–1.40, P=0.75, respectively). Moreover, a strong association with GO was present in males (OR=2.06, 95% CI=1.40–3.02, P=0.0002), whereas it was absent in females (OR=1.10, 95% CI=0.90–1.35, P=0.35). The results of our study further suggest that rs11675434 SNP located near TPO is associated with the development of GO, especially in males and patients with later age of GD onset.
Similar content being viewed by others
Introduction
The thyroid peroxidase (TPO) is a key enzyme of thyroid function, having a pivotal role in the synthesis of thyroid hormones. Whereas multiple studies showed that various TPO gene mutations may cause dysfunction of the TPO enzyme in patients with congenital hypothyroidism,1, 2 the role of TPO gene polymorphisms in the susceptibility to autoimmune thyroid disease remains unclear. Interestingly, single-nucleotide polymorphisms (SNPs) near TPO have been recently associated with TPO antibody (TPOAb) serum levels in two independent population-based genome-wide association studies conducted in Caucasian and Asian populations.3, 4 As the appearance of TPOAb often precedes the development of an overt autoimmune thyroid disease, including Graves’ disease (GD), we assessed the association of the most significantly associated SNP in the TPO region identified in the genome-wide association study by Medici et al.3 (rs11675434) with susceptibility to and phenotype of GD in a cohort of GD patients from the Polish population.5 Although rs11675434 was not associated with susceptibility to GD, we observed a strong association between this SNP and the presence of clinically evident Graves’ ophthalmopathy (GO).5 Taking into consideration the emerging evidence for gender-dependent differences in effects of genetic polymorphisms on the regulation of thyroid function,6 and gene–environment interactions in patients with late and early onset of the disease resulting from differing lengths of exposure to environmental factors,7, 8 the aim of the current study was to reevaluate the association between rs11675434 and GO in an extended group of well-characterized patients with GD, as well as to assess the differences in gender-dependent and age-of-onset-specific effects of the studied SNP.
Materials and methods
Subjects
A total of 1231 GD patients from the Polish Caucasian population, including 1043 adults (827 females, 216 males) and 188 unrelated children and adolescents (149 females, 39 males) with a confirmed diagnosis of GD were included in the study. The adult patients were recruited from three independent cohorts: Warsaw, Gliwice and Wroclaw, whereas children and adolescent patients were recruited mainly from the Bialystok cohort; all cohorts have been described in previous studies.8, 9, 10, 11 The diagnosis of GD was established on the basis of clinical and biochemical symptoms of hyperthyroidism accompanied by diffuse goiter, detectable TSH receptor autoantibodies and/or increased radioiodine uptake, as described in the previous studies.8, 9, 10, 11 The severity of the GO was assessed using NOSPECS classification,12 and clinically evident GO was defined as: proptosis, extraocular muscle dysfunction, exposure keratitis and optic neuropathy (NOSPECS ⩾3, moderate-to-severe and sight-threatening ophthalmopathy basing on EUGOGO consensus13). The clinical characteristics of the study group are shown in Table 1.
The control group consisted of 970 healthy adults (730 females, 240 males) and 160 unrelated healthy children and adolescents (75 females, 85 males), and comprised cohorts used in the previous studies.8, 9, 10
Written informed consent was collected from all of the participants and the local ethical committees of the participating institutions approved the study.
Genotyping
The analyzed SNP was genotyped using a real-time PCR method with a predesigned TaqMan SNP Genotyping Assay provided by Applied Biosystems, Woolston, Warrington, UK. The genotyping procedure was performed according to the manufacturer’s protocol on the QuantStudioTM 12K Flex instrument (Life Technologies Corporation, Carlsbad, CA, USA). The overall genotyping call rate was 96.65%.
Statistical analysis
P<0.05 was considered statistically significant. The genotype distributions of the analyzed SNP were tested for Hardy–Weinberg equilibrium and showed no evidence of deviation (P=0.80 and P=0.77 in the total group of patients and controls, respectively). The χ2-test showed no significant difference in the genotype distributions within the analyzed cohorts of GD patients (P=0.42) and they were analyzed together.
Allele frequencies and genotype distributions in the whole group of GD patients and controls were compared using the χ2-test. The genotype distributions were analyzed assuming different models of inheritance (dominant, additive and recessive). The association with susceptibility to GD was also analyzed separately for subgroups of GD patients stratified on the basis of gender (males, females) and age of GD onset (children and adolescents with GD onset ⩽18 years, younger adults with GD onset 18–45 years and older adults with GD onset ⩾45 years).
A similar comparison of allele frequencies and genotype distributions with subgroup analysis was subsequently performed between GD patients with clinically evident GO (NOSPECS class 3–6) and without clinically evident GO (NOSPECS class 0–1), as well as healthy controls. As GD patients with a milder phenotype of ophthalmopathy, characterized by involvement of only the soft tissue (NOSPECS class 2), tend to be variously classified in different studies, which has a negative impact on the reliability of the results obtained, they were excluded from the current analyses.
Web-Assotest (http://www.ekstroem.com/assotest/assotest.html) and Statistica (StatSoft, Tulsa, OK, USA) software were used for the analyses.
Results
Association with GD
In the allele analysis, rs11675434 showed no association with susceptibility to GD in the total group of analyzed subjects (odds ratio (OR)=1.09, 95% confidence interval (CI)=0.97–1.22, P=0.16). Neither further subgroup analyses revealed any gender-dependent or age-of-onset-specific effects of the studied SNP on GD susceptibility (Table 2). The study had power (alpha=0.05, power=0.8) to detect allelic OR=1.18, considering alleles prevalence in the control group (minor allele frequency, (MAF)=0.41) and the total number of subjects analyzed. Likewise, there was no significant difference in the genotype distribution at rs11675434 between the total group of GD patients and controls, nor in any subgroup of GD patients analyzed separately (Supplementary Table 1).
Association with GO
On the contrary, the T allele was significantly more frequent in patients with than without clinically evident GO (OR=1.26, 95% CI=1.05–1.51, P=0.012), which was consistent with our previous findings.5 Further analyses performed in subgroups of patients showed that the association with GO was borderline significant in older adults (OR=1.34, 95% CI=1.03–1.75, P=0.031), but not in children and adolescents (OR=1.72, 95% CI=0.77–3.84, P=0.18) or in younger adults (OR=1.05, 95% CI=0.79–1.40, P=0.75). Moreover, a strong association was present in males (OR=2.06, 95% CI=1.40–3.02, P=0.0002), whereas it was absent in females (OR=1.10, 95% CI=0.90–1.35, P=0.35; Table 3). Similarly, the genotype distributions at rs11675434 were significantly different in GD patients with and without GO in the total group of patients, whereas in subgroup analysis, a significant difference was observed in males and older adults (Supplementary Table 2). We did not find any significant difference in the allele/genotype distribution at rs11675434 between patients with moderate-to-severe and sight-threatening ophthalmopathy (data not shown). When we compared subsets of GO patients with healthy controls, we confirmed a significant difference in allele distributions in the total group of GO patients (Table 4, OR=1.31, 95% CI=1.11–1.54, P=0.0014), as well as in GO subsets of males and adult patients with later age of GD onset (OR=1.58, 95% CI=1.17–2.15, P=0.0031 and OR=1.39, 95% CI=1.13–1.70, P=0.0017, respectively). Although we also observed a borderline significant difference in females (OR=1.23, 95% CI=1.03–1.48, P=0.024), this association was absent when we compared subsets of female GD patients with and without GO. The differences in genotype distributions at rs11675434 between GO patients and controls are shown in Supplementary Table 3. Importantly, if a conservative Bonferroni’s correction adjusted for a number of tests performed was applied, the only significant association between the studied SNP and GO in our study was found between male GD patients with and without GO (P=0.0002, Pcor=0.014).
Discussion
The results of our study further suggest that rs11675434 SNP located near TPO may be associated with the development of clinically evident GO, especially in males and patients with later age of GD onset.
Being located on the apical membrane of thyrocytes, the TPO enzyme is responsible for the iodination of the tyrosine residues in thyroglobulin, and coupling of the iodinated tyrosines to generate triiodothyronine (T3) and thyroxine (T4).14 It is a large glycoprotein encoded by the TPO gene located on chromosome 2, consisting of over 150 kb and 17 exons.15 rs11675434 is a SNP located in the upstream region of the TPO gene (NBCI build 36, chr2:1386822). Kwak et al.4 showed that rs2071403, a SNP in linkage disequilibrium with rs11675434 (r2=0.509, D′=1.00 in CEU population), is associated with the TPOAb serum levels, as well as with the TPO messenger RNA levels in human thyroid tissue. Several studies suggested that TPOAb may take part in the antibody-dependent thyrotoxicity in autoimmune thyroid disease patients in both cell-mediated and complement-dependent mechanisms.16, 17 Interestingly, the TPO gene expression in retrobulbar tissue in both GD patients and healthy subjects have been reported.18 Although a predominant role of the autoantibodies against the thyroid-stimulating hormone receptor (TRAb) in the pathogenesis of GO seems to be established,19, 20, 21 the TPOAb-dependent cytotoxicity mechanisms may be also involved in the process, potentially explaining the presence of GO symptoms in TRAb-negative patients. However, the association between TPOAb and GO is still questionable, as the results of previous studies on this issue are highly inconsistent. Whereas it was reported that the lower TPOAb serum levels are associated with an increased risk of GO development in GD patients,22 other studies yielded the opposite results23 or negated the association.24 Therefore further studies on the pathogenesis of GO are needed to clarify the role of TPOAb, and explain the underlying mechanisms of the observed association between rs11675434 and GO. As ocular complications may significantly reduce the quality of life in GD patients,25 identifying ones with high risk of developing severe ophthalmopathy before the complications occur could also allow clinicians to provide patients with an early, adjusted and thus more effective treatment.
Some interesting findings of our study are the gender-dependent and age-of-onset-specific differences in the observed effects of the studied SNP. Although their underlying mechanisms remain unclear, these results correspond with the gender-dependent differences in effects of genetic polymorphisms on the regulation of thyroid function reported recently by Porcu et al.6 Also the results of our recent research on the association between the TSHR gene polymorphism and the susceptibility to GO suggested a significant association in patients with early onset of GD, which was not observed in patients with later onset of the disease, nor in the total group of GD patients analyzed in the study.26 Whereas twin studies clearly indicate that the genetic predisposition has the major role in GD,27 still only ~10% of the genetic susceptibility can be explained based on the loci identified in previous studies, including genome-wide association studies.28 Therefore, improved phenotyping and use of precise phenotypes in genetic association studies may provide a better insight into the genetic background of the disease.29 As the results of our study support that hypothesis, we strongly recommend to include genotype–phenotype association analyses in further studies. On the other hand, multiple testing used for subsets analyses may increase the risk of false-positive findings. If a conservative Bonferroni’s correction was applied, the association between the studied SNP and GO in our study remained significant in male patients only. Therefore, our results require confirmation in other cohorts.
References
Ris-Stalpers, C. & Bikker, H. Genetics and phenomics of hypothyroidism and goiter due to TPO mutations. Mol. Cell. Endocrinol. 322, 38–43 (2010).
Belforte, F. S., Miras, M. B., Olcese, M. C., Sobrero, G., Testa, G., Munoz, L. et al. Congenital goitrous hypothyroidism: mutation analysis in the thyroid peroxidase gene. Clin. Endocrinol. 76, 568–576 (2012).
Medici, M., Porcu, E., Pistis, G., Teumer, A., Brown, S. J., Jensen, R. A. et al. Identification of novel genetic loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLoS Genet. 10, e1004123 (2014).
Kwak, S. H., Park, Y. J., Go, M. J., Lee, K. E., Kim, S. J., Choi, H. S. et al. A genome-wide association study on thyroid function and anti-thyroid peroxidase antibodies in Koreans. Hum. Mol. Genet. 23, 4433–4442 (2014).
Kus, A., Szymanski, K., Peeters, R. P., Miskiewicz, P., Porcu, E., Pistis, G. et al. The association of thyroid peroxidase antibody risk loci with susceptibility to and phenotype of Graves' disease. Clin. Endocrinol. 83, 556–562 (2015).
Porcu, E., Medici, M., Pistis, G., Volpato, C. B., Wilson, S. G., Cappola, A. R. et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet. 9, e1003266 (2013).
Tomer, Y., Menconi, F., Davies, T. F., Barbesino, G., Rocchi, R., Pinchera, A. et al. Dissecting genetic heterogeneity in autoimmune thyroid diseases by subset analysis. J. Autoimmun. 29, 69–77 (2007).
Jurecka-Lubieniecka, B., Ploski, R., Kula, D., Krol, A., Bednarczuk, T., Kolosza, Z. et al. Association between age at diagnosis of Graves' disease and variants in genes involved in immune response. PLoS ONE 8, e59349 (2013).
Skorka, A., Bednarczuk, T., Bar-Andziak, E., Nauman, J. & Ploski, R. Lymphoid tyrosine phosphatase (PTPN22/LYP) variant and Graves' disease in a Polish population: association and gene dose-dependent correlation with age of onset. Clin. Endocrinol. 62, 679–682 (2005).
Bossowski, A., Borysewicz-Sanczyk, H., Wawrusiewicz-Kurylonek, N., Zasim, A., Szalecki, M., Wikiera, B. et al. Analysis of chosen polymorphisms in FoxP3 gene in children and adolescents with autoimmune thyroid diseases. Autoimmunity 47, 395–400 (2014).
Pawlak-Adamska, E., Daroszewski, J., Bolanowski, M., Oficjalska, J., Janusz, P., Szalinski, M. et al. PPARg2 Ala(1)(2) variant protects against Graves' orbitopathy and modulates the course of the disease. Immunogenetics 65, 493–500 (2013).
Werner, S. C. Modification of the classification of the eye changes of Graves' disease: recommendations of the Ad Hoc Committee of the American Thyroid Association. J. Clin. Endocrinol. Metab. 44, 203–204 (1977).
Bartalena, L., Baldeschi, L., Dickinson, A. J., Eckstein, A., Kendall-Taylor, P., Marcocci, C. et al. Consensus statement of the European group on Graves' orbitopathy (EUGOGO) on management of Graves' orbitopathy. Thyroid 18, 333–346 (2008).
McLachlan, S. M. & Rapoport, B. The molecular biology of thyroid peroxidase: cloning, expression and role as autoantigen in autoimmune thyroid disease. Endocr. Rev. 13, 192–206 (1992).
Kimura, S., Hong, Y. S., Kotani, T., Ohtaki, S. & Kikkawa, F. Structure of the human thyroid peroxidase gene: comparison and relationship to the human myeloperoxidase gene. Biochemistry 28, 4481–4489 (1989).
Rodien, P., Madec, A. M., Ruf, J., Rajas, F., Bornet, H., Carayon, P. et al. Antibody-dependent cell-mediated cytotoxicity in autoimmune thyroid disease: relationship to antithyroperoxidase antibodies. J. Clin. Endocrinol. Metab. 81, 2595–2600 (1996).
Rebuffat, S. A., Nguyen, B., Robert, B., Castex, F. & Peraldi-Roux, S. Antithyroperoxidase antibody-dependent cytotoxicity in autoimmune thyroid disease. J. Clin. Endocrinol. Metab. 93, 929–934 (2008).
Lai, O. F., Zaiden, N., Goh, S. S., Mohamed, N. E., Seah, L. L., Fong, K. S. et al. Detection of thyroid peroxidase mRNA and protein in orbital tissue. Eur. J. Endocrinol. 155, 213–218 (2006).
Feliciello, A., Porcellini, A., Ciullo, I., Bonavolonta, G., Avvedimento, E. V. & Fenzi, G. Expression of thyrotropin-receptor mRNA in healthy and Graves' disease retro-orbital tissue. Lancet 342, 337–338 (1993).
Paschke, R., Vassart, G. & Ludgate, M. Current evidence for and against the TSH receptor being the common antigen in Graves' disease and thyroid associated ophthalmopathy. Clin. Endocrinol. 42, 565–569 (1995).
Eckstein, A. K., Plicht, M., Lax, H., Neuhauser, M., Mann, K., Lederbogen, S. et al. Thyrotropin receptor autoantibodies are independent risk factors for Graves' ophthalmopathy and help to predict severity and outcome of the disease. J. Clin. Endocrinol. Metab. 91, 3464–3470 (2006).
Khoo, D. H., Ho, S. C., Seah, L. L., Fong, K. S., Tai, E. S., Chee, S. P. et al. The combination of absent thyroid peroxidase antibodies and high thyroid-stimulating immunoglobulin levels in Graves' disease identifies a group at markedly increased risk of ophthalmopathy. Thyroid 9, 1175–1180 (1999).
Lee, J. H., Park, S. H., Koh, D. G. & Suh, B. K. Thyroid peroxidase antibody positivity and triiodothyronine levels are associated with pediatric Graves' ophthalmopathy. World J. Pediatr. 10, 155–159 (2014).
McLachlan, S. M., Bahn, R. & Rapoport, B. Endocrine ophthalmopathy: a re-evaluation of the association with thyroid autoantibodies. Autoimmunity 14, 143–148 (1992).
Sawicka-Gutaj, N., Bednarczuk, T., Daroszewski, J., Waligorska-Stachura, J., Miskiewicz, P., Sowinski, J. et al. GO-QOL–-disease-specific quality of life questionnaire in Graves' orbitopathy. Endokrynol. Pol. 66, 362–366 (2015).
Jurecka-Lubieniecka, B., Ploski, R., Kula, D., Szymanski, K., Bednarczuk, T., Ambroziak, U. et al. Association between polymorphisms in the TSHR gene and Graves' orbitopathy. PLoS ONE 9, e102653 (2014).
Brix, T. H., Kyvik, K. O., Christensen, K. & Hegedus, L. Evidence for a major role of heredity in Graves' disease: a population-based study of two Danish twin cohorts. J. Clin. Endocrinol. Metab. 86, 930–934 (2001).
Chu, X., Pan, C. M., Zhao, S. X., Liang, J., Gao, G. Q., Zhang, X. M. et al. A genome-wide association study identifies two new risk loci for Graves' disease. Nat. Genet. 43, 897–901 (2011).
Manolio, T. A., Collins, F. S., Cox, N. J., Goldstein, D. B., Hindorff, L. A., Hunter, D. J. et al. Finding the missing heritability of complex diseases. Nature 461, 747–753 (2009).
Acknowledgements
The study was supported by National Science Center grant 2014/15/N/NZ5/01656. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Kuś, A., Szymański, K., Jurecka-Lubieniecka, B. et al. Gender-dependent and age-of-onset-specific association of the rs11675434 single-nucleotide polymorphism near TPO with susceptibility to Graves’ ophthalmopathy. J Hum Genet 62, 373–377 (2017). https://doi.org/10.1038/jhg.2016.135
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2016.135
This article is cited by
-
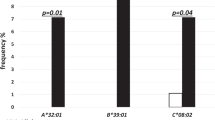
Significance of HLA in the development of Graves’ orbitopathy
Genes & Immunity (2023)
-
The risk factors for Graves’ ophthalmopathy
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)