Abstract
It has been reported that organic anion-transporting polypeptide (OATP) 1B1, OATP1B3 and multidrug resistance-associated protein 2 are involved in the hepatobiliary transport of olmesartan. We investigated the association of SLCO1B1, SLCO1B3 and ABCC2 polymorphisms with the pharmacokinetics of olmesartan. We sequenced all exons, exon–intron junctions and the 5′ and 3′ flanking regions of the three genes in 115 individuals from African-American, Hispanic and Caucasian populations who had participated in our clinical studies. A total of 348 single-nucleotide polymorphisms (SNPs) were identified with a minor allele frequency of ⩾0.01 in at least one population; 132 SNPs were detected in SLCO1B1, 130 in SLCO1B3 and 86 in ABCC2. We characterized the linkage disequilibrium (LD) and haplotypes shared across the populations and then evaluated the association between the haplotypes and the pharmacokinetics of olmesartan. Seven inter-ethnic LD blocks were observed in SLCO1B1, while three in SLCO1B3 and four in ABCC2. Although extensive variability in the sequences of SLCO1B1, SLCO1B3 and ABCC2 existed across the three populations, there was no remarkable difference in any pharmacokinetic parameters of olmesartan between subjects with and without any major haplotypes in the three transporter genes we tested.
Similar content being viewed by others
Introduction
Olmesartan medoxomil, a potent angiotensin II type 1 receptor antagonist, is orally administered as a prodrug and completely hydrolyzed into the active metabolite olmesartan during absorption from the gastrointestinal tract. Olmesartan is excreted into both bile and urine without undergoing metabolism.1 Some studies have suggested that organic anion-transporting polypeptide 1B1 (gene name, SLCO1B1) and organic anion-transporting polypeptide 1B3 (SLCO1B3) are involved in hepatic uptake and that the ATP-binding cassette, sub-family C member 2 (multidrug resistance-associated protein (MRP) 2, ABCC2), is involved in the biliary excretion of olmesartan.2, 3
Membrane transporters have important roles in the uptake, distribution and excretion of endogenous compounds and xenobiotics. Polymorphisms in genes encoding transporters have been investigated extensively and appear to be associated with altered transporter activity. It has become clear that genetic polymorphisms have an impact on the inter-individual variability of the pharmacokinetics and pharmacodynamics of drugs. Indeed, the 521T>C (V174A) variant of SLCO1B1 is associated with changes in the transporter activity of estrone sulfate, 17β-D-glucuronide and pravastatin in vitro.4, 5, 6, 7 Moreover, the 521C/C genotype has been associated with increased plasma concentrations of statins and glinides in human studies.8, 9, 10 In SLCO1B3, several single-nucleotide polymorphisms (SNPs) have been identified in coding and noncoding regions. At least three nonsynonymous SNPs have been found so far, with 334T>G (S112A) and 699G>A (M233I) increasing transporter activity in vitro.11 With regard to ABCC2, several mutations and deletions have been identified in patients with Dubin–Johnson syndrome, an autosomal recessive disorder, each of which impairs either the expression or function of MRP2 protein.12, 13, 14 Beside mutations in Dubin–Johnson syndrome, the extensive genetic variation identified so far may have a potential effect on drug disposition.15, 16 In fact, the T allele of −24C>T has been associated with lower mRNA levels in normal renal tissues, leading to reduced transporter activity.17 In addition, the synonymous mutation 1446C>G was associated with increased hepatic mRNA expression resulting in low Cmax and area under the plasma concentration time curve (AUC) values for pravastatin.18
A haplotype-based approach to identifying the genetic variation underlying drug response and disposition as well as common diseases has been proposed.19, 20, 21, 22 The International HapMap Project has characterized the pattern of haplotype structure and linkage disequilibrium (LD) across the human genome to facilitate genome-wide association studies.23, 24 It is well established that, in high LD regions, only a limited number of haplotypes is observed and common haplotypes can be efficiently labeled with a small number of common SNPs, haplotype-tagging SNPs. These can maintain most of the information for the detection of other variants, while reducing the amount of genotyping. The use of haplotypes in association studies may have advantages over the use of individual SNPs. For example, if the causal allele is dependent on cis interactions with the alleles of other SNPs, the association may not be revealed unless the haplotype is evaluated.25, 26 Furthermore, haplotypes in a genomic region of interest can serve as genetic markers to detect an association with the phenotype, whether or not the markers themselves have a causal effect.
In this study, we investigated the distribution of SNPs of SLCO1B1, SLCO1B3 and ABCC2 involved in the hepatobiliary transport of olmesartan and estimated common LD blocks and common haplotypes across three ethnic/racial populations. We used a haplotype-based approach to assess the influence of haplotypes of these transporters on the pharmacokinetics of olmesartan.
Materials and Methods
Subjects
There were 120 subjects who had participated in two of our studies on AZOR (combination tablet formulation of olmesartan medoxomil and amlodipine besylate). Their characteristics are listed in Table 1. Ages ranged from 18 to 45 years, and 42 subjects were female. Ethnicity was classified based on a self-description. In all, 40 of the subjects classified themselves as African-American, 17 as Caucasian, 61 as Hispanic and 2 as other. Owing to low DNA yields from 5 subjects, a total of 115 subjects were used. The health status of each individual was confirmed based on their medical history, a physical examination, laboratory reports including hematology and serum chemistry, and a 12-lead electrocardiogram. Clinical studies were conducted at MDS Pharma Services (Nepture, NZ and Phoenix, AZ, USA). The protocols were approved by the Institutional Review Boards of the study center and all the subjects gave written informed consent before admission into the studies. The protocols were conducted in accordance with the guidelines on Good Clinical Practice and with ethical standards for human experimentation established by the Declaration of Helsinki Principles.
Study design
Study 1 was a phase I bioavailability study with parallel-group, open-label, randomized and crossover designs. Two cohorts (cohort 1 and cohort 2) of 30 subjects each were enrolled in sequential order, with the first 30 subjects assigned to cohort 1 and the second 30 subjects to cohort 2. The subjects in cohort 1 were randomized to receive single doses of treatment A (AZOR: combination tablet formulation of olmesartan medoxomil 10 mg and amlodipine besylate 5 mg) or treatment B (olmesartan medoxomil 10 mg co-administered with amlodipine besylate 5 mg) in one of two sequences (AB or BA). In cohort 2, the subjects were randomized to receive single doses of treatment C (AZOR: combination tablet formulation of olmesartan medoxomil 40 mg and amlodipine besylate 10 mg) or treatment D (olmesartan medoxomil 40 mg co-administered with amlodipine besylate 10 mg) in one of two sequences (CD or DC). A 21-day-washout period followed the dosing for each period.
Study 2 was a phase I study with parallel-group, open-label, randomized, single-dose and 3-period crossover designs. Again two cohorts were established. The subjects in cohort 1 were randomized to receive single doses of treatment A (AZOR: combination tablet formulation of olmesartan medoxomil 40 mg and amlodipine besylate 10 mg), treatment B (AZOR: combination tablet formulation of olmesartan medoxomil 20 mg and amlodipine besylate 5 mg) or treatment C (AZOR: combination tablet formulation of olmesartan medoxomil 10 mg and amlodipine besylate 10 mg) in one of six possible treatment sequences. In cohort 2, the subjects were randomized to receive single doses of treatment D (AZOR: combination tablet formulation of olmesartan medoxomil 40 mg and amlodipine besylate 5 mg), treatment E (AZOR: combination tablet formulation of olmesartan medoxomil 20 mg and amlodipine besylate 10 mg) or treatment F (AZOR: combination tablet formulation of olmesartan medoxomil 10 mg and amlodipine besylate 5 mg) in one of six possible treatment sequences. A 21-day-washout period followed the dosing for each period.
Sample treatments and pharmacokinetic data analysis
Blood samples were collected into EDTA blood collection tubes and plasma was separated by centrifugation. Two aliquots of at least 0.7 ml of plasma each were removed, placed in screw-cap polypropylene tubes and immediately stored at −20 °C or below until the analysis. Blood samples were collected before and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, 24, 36, 48, 60 and 72 h following each dose. The plasma concentrations of olmesartan were determined by a validated method of high-performance liquid chromatography with fluorescence detection. The lower limit of quantification for olmesartan was 1 ng ml−1.27 The pharmacokinetic parameters were calculated from the individual plasma concentrations of olmesartan by non-compartmental methods using WinNonlin Professional, version 4.0.1 (Pharsight, St Louis, MO, USA).
Identification of SNPs and genotyping
For genotyping, blood samples were collected into polypropylene EDTA evacuated tubes before the first treatment in each study. Genomic DNA was isolated from leukocytes. All of the exons, exon–intron junctions and 2 kb of the 5′ and 3′ flanking regions of SLCO1B1, SLCO1B3 and ABCC2 in the DNA from 115 subjects were amplified for the sequencing. Figure 1 illustrates the regions of each gene sequenced. The primers are summarized in Tables 1–3 (Supplementary data). PCR was performed in a GeneAmp PCR System 9700 (Applied Biosystems, Carlsbad, CA, USA). All the amplicons were purified, diluted and used in standard Big-Dye terminator sequence reactions under standard procedures (Applied Biosystems), and the sequence was determined on an ABI 3730xL automated sequencer (Applied Biosystems). Each chromatogram was assembled in the NCBI human genome reference sequences NT_009714.16 for SLCO1B1 and SLCO1B3 and NT_030059.12 for ABCC2 using the Phred/Phrap/Consed28, 29, 30 software suite. Only putative SNPs were detected in the sequenced regions by the PolyPhred, version 4.0.31 In contrast, insertion/deletion (indel) variations were not selected as candidate loci to construct an LD block, because the indel search function of PolyPhred version 4.0 was still under development and identified only sequences that appeared to be heterozygous for an indel. The positions of the SNPs were given in relation to the NCBI references NM_006446.2 for SLCO1B1, NM_019844.1 for SLCO1B3 and NM_000392.1 for ABCC2.
SNPs and LD blocks of SLCO1B1 (a), SLCO1B3 (b) and ABCC2 (c).The horizontal line depicts the genomic region and the blue boxes show the exons of the transporter genes. Below the line, all the identified SNPs and haplotype tagged SNPs (htSNPs) are indicated in orange and red, respectively. For LD structure, SNPs with MAF⩾0.1 were used in each population. Red, yellow and blue bars indicate the LD blocks of the African-American, Hispanic and Caucasian populations, respectively. Inter-ethnic LD blocks are presented in pink at the bottom of the figures.
Statistical analysis
Deviations from the Hardy–Weinberg equilibrium were tested with the SNP-HWE software32 using an exact test. LD for each pair of SNPs was quantified by D’ values and LD block structures were determined using the program QTLHAPLO.33 Estimation of haplotype frequencies and identifying haplotype-tagging SNPs were performed by the same program. Haplotype marker-trait associations were tested with a mixed model using nlm package in R34 to handle unbalanced and correlated observations arising from repeated measures on an individual who was administered with olmesartan at various dosages, 10, 20 and 40 mg. The model contained subjects as a random effect, diplotype as the predictor of main interest and other fixed effects including doses of olmesartan medoxomil and creatinine clearance values that were calculated with the Cockcroft-Gault formula.35 However, ethnicity was not included in the final model, as a result of the prior evaluation using the model selection criterion Akaike’s information criterion. The pharmacokinetic data, AUC0−t and Cmax were log-transformed for the statistical analysis. The significance level for the association tests was set at P<0.05 after multiple testing correction by the Benjamini and Hochberg method36 in each genetic inheritance mode (trend, genotype, dominant or recessive).
Results
Allele frequencies of SNPs
A total of 348 SNPs were identified; a SNP was defined as a genotype success rate exceeding 80% and with a minor allele frequency (MAF) of ⩾0.01 in at least one population group. There was no evidence of deviation from the Hardy–Weinberg equilibrium within each population. The allele frequencies, locations and identifier codes for SNPs are shown in Table 2a–c. A total of 132 SNPs were detected in SLCO1B1, 130 in SLCO1B3 and 86 in ABCC2. The rates of newly detected SNPs within these were ∼0.1%, 0.07% and 0.3%, respectively. The majority of the SNPs were located in introns; with only 54 detected in coding regions, including 33 nonsynonymous SNPs.
The allele frequencies of some SNPs varied among populations (Table 2). A total of 149 population-specific SNPs were observed (149/348). African-Americans had the highest number of population-specific SNPs, 131, while Hispanics had 10 and Caucasians 8. Meanwhile, some SNPs were detected across the three populations with MAF⩾0.05; 36 SNPs in SLCO1B1, 48 in SLCO1B3 and 12 in ABCC2.
We evaluated LD using SNPs with a MAF⩾0.1 in each population by QTLHAPLO. We defined an LD block as a consecutive region with D′>0.9 and extended an edge of the LD block by using an adjacent SNP while the cumulative frequency of major haplotype with a frequency>0.1 exceeded 0.9. In SLCO1B1, 12 LD blocks were inferred from 38 SNPs in African-Americans, 8 were inferred from 36 SNPs in Hispanics and 6 were inferred from 29 SNPs in Caucasians (Figure 1). In SLCO1B3, 11 LD blocks were inferred from 57 SNPs in African-Americans, 5 were inferred from 47 SNPs in Hispanics and 3 were inferred from 19 SNPs in Caucasians. In ABCC2, 5 LD blocks were inferred from 18 SNPs in African-Americans, 3 were inferred from 12 SNPs in Hispanics and 2 were inferred from 9 SNPs in Caucasians. The number of LD blocks differed across the three populations, with the African-American population having a larger number and shorter stretches of LD in SLCO1B1 and SLCO1B3.
Inter-ethnic LD blocks and haplotypes based on tag SNPs
In an attempt to define the inter-ethnic LD blocks across the populations, we examined two population-based (Hispanics and Caucasians) LD blocks by QTLHAPLO. Block structure was assessed using SNPs with a MAF⩾0.1 and any overlapping pairs were joined.19 Borders were characterized by the SNPs at the terminal ends of the block in any one population. We did not adapt the LD block structure of African-Americans, due to the small block size compared to the other populations. Figure 1 also illustrates the inter-ethnic LD blocks of the three genes. Eight inter-ethnic LD blocks were observed in SLCO1B1; block 1 (SNP ID 574∼485), block 2 (485∼514), block 3 (493∼521), block 4 (514∼525), block 5 (514∼546), block 6 (546∼592) and block 7 (592∼616). In SLCO1B3, three inter-ethnic LD blocks were observed; block 1 (633∼666), block 2 (646∼684) and block 3 (666∼765). ABCC2 contained four inter-ethnic LD blocks; block 1 (792∼796), block 2 (792∼867), block 3 (795∼891) and block 4 (848∼891).
The haplotypes in the inter-ethnic LD blocks were estimated using SNPs with a MAF⩾0.05. The tagged SNPs were selected from the Hispanic and Caucasian population-based LD blocks. We merged the haplotype tagged SNPs from any one population as the inter-ethnic tagged SNPs, after identifying the haplotype tagged SNPs in each population using QTLHAPLO. The most frequent haplotypes in each block for the three genes are summarized in Table 3. In general, African-Americans tended to have greater diversity than the Hispanic and Caucasian populations. The most frequent haplotypes in each block were almost the same for Hispanics and Caucasians, but different for African-Americans.
Association study
Table 4 shows the results of the association study between SLCO1B1, SLCO1B3 and ABCC2 haplotypes and the pharmacokinetics of olmesartan. The mean and s.d. of pharmacokinetic parameters of olmesartan for 2-copy carriers, 1-copy carriers and noncarriers for the major haplotypes in SLCO1B1, SLCO1B3 and ABCC2 are shown in Table 4 to 6 (Supplementary data). However, the pharmacokinetic data of treatment B and D in cohort 1 were not used because of the difference of the formulation. After statistical analysis, some haplotypes showed association with pharmacokinetic parameters in SLCO1B1 and SLCO1B3. In SLCO1B1, allele dosage effects (P=0.0042, 0.0093) in all dose groups in two haplotypes (AGAAA and ACCTTC) were shown. In SLCO1B3, the smallest P-value (P=0.0153) was shown in the GACCT haplotype in block 3. However, none of these results pass the significance threshold after multiple test correction. On the other hand, there was no haplotype-trait association in the ABCC2 gene.
Discussion
The difference of the length of LD blocks is observed across ethnic groups. Hispanics and African-Americans are also known as racially admixed populations. We therefore thought that the genetic backgrounds of our Hispanic and African-American samples might be different from those of the HapMap MXL and ASW. In this study, we attempted to discover SNPs and define putative LD blocks in three ethnic populations, African-American, Hispanic and Caucasian, considering the nature of the LD across SLCO1B1, SLCO1B3 or ABCC2 genes to identify inter-ethnic LD blocks. Among 348 SNPs we identified in our study, 149 SNPs (42.8%) were population-specific SNPs; 131 for African-Americans, 10 for Hispanics and 8 for Caucasians, indicating that large intra-ethnic diversity in frequency of SNPs in African-Americans. In contrast, 133 SNPs (38.2%) were detected across the three populations; 49 SNPs (37.1%, 49/132 in total) for SLCO1B1, 63 (48.5%) for SLCO1B3 and 21 (24.4%) for ABCC2, suggesting that large inter-ethnic differences in frequency of SNPs in ABCC2 among the three genes we investigated.
In SLCO1B1, we defined 12 major haplotypes in 7 common LD blocks. Carriers with AGAAA haplotype in block 2 or GAAT haplotype in block 3 tended to show higher AUC values than noncarriers. Both AGAAA and GAAT haplotypes include SNP 505 (underlined), registered in dbSNP as rs2306283 and located in exon 5, which was reported as the functionally important SNP 388A>G (N130D).37, 38 Two human studies on the pharmacokinetic profile of pravastatin reported that 388A>G was associated with increased transport activity of organic anion-transporting polypeptide 1B1, leading to lower AUC of pravastatin in subjects having G allele position at 505.37, 38 Higher AUC values in subjects with A allele may be due to SNP 388A>G; however, these trends were not statistically significant. Another functionally important SNP is 521T>C (rs4149056; V174A), SNP 524 in block 4.8 In our study, SNP 524 was not associated with changes in any pharmacokinetic parameters. In addition to this, the SLCO1B1*1A (388A–521C), *1B (388G–521T), *5 (388A–521C) and *15 (388G–521C) haplotypes,4 which were formed through these two SNPs, did not show any statistical significant associations (data not shown).
With regard to SLCO1B3, we defined six major haplotypes. Although GACCT haplotype in block 3 was associated with changes in the pharmacokinetics of olmesartan, there was no significant association after multiple test correction. TGATC haplotype in block 3 showed allele dosage trends in all dose groups. As the frequency of TGATC haplotype in African-Americans was the highest (0.59 vs 0.19 and 0.10) in the three populations, lower AUC values are expected in this population even when the same dosage is administered. All SNPs, 666, 684, 693, 730 and 740, composing both haplotype were located in intron regions. Thus, reasons to why this haplotype is associated with changes in pharmacokinetics of olmesartan are not cleared. However, interestingly, there were 32 SNPs that showed a high LD (r2>0.8 in all populations) relationship with SNP 693, including the cSNPs at 683 (334T>G, S112A, rs4149117 in exon 4), 721 (699G>A, M233I, rs7311358 in exon 7) and 757 (1557A>G, A519A, rs2053098 in exon 12) (data not shown).
In our study, SNPs 683 and 721 also showed a strong LD (r2=0.94, 0.81 and 1.0 in African-Americans, Hispanics and Caucasians, respectively). Studies on the pharmacogenomics of SLCO1B3 are scant in comparison with those on SLCO1B1. The association between SLCO1B3 polymorphisms and the pharmacokinetics of paclitaxel has been studied; however, the clearance of unbound paclitaxel was not significantly associated with the SLCO1B3 334T>G or 699G>A polymorphisms.39 Functional impacts of these SNPs on substrate drugs are warranted to be cleared in humans.
In ABCC2, no haplotype showed significantly associations with any pharmacokinetics parameters of olmesartan. We defined four common LD blocks in this study. Block 1 is located in the 5′ flanking region of ABCC2, and SNP 796 in block 1, which corresponds to SNP –24C>T, has been shown to reduce mRNA expression.17 Several studies have examined the functional meaning of the coding SNPs in ABCC2, but there was no obvious genetic link between the coding SNPs and the haplotype in our study.
The single-SNP-based analysis was also conducted for all haplotype tagged SNPs to discuss the effect of individual SNP on the olmesartan pharmacokinetics, but there was no significant result after multiple test correction (Table 7, Supplementary data).
It has been reported that olmesartan has a dual clearance pathway, with ∼60% of a dose being excreted into feces via bile and the remaining 40% being excreted into the urine,1, 2 and that multiple transporters in multiple tissues were involved in the overall pharmacokinetics of olmesartan.3 Therefore, the effect of genetic variations of single transporters on the pharmacokinetics of olmesartan is considered to be small.
In the association study using subjects in early phase clinical studies, the sample size of enrolled subjects is often insufficient. Based on a two-tailed t-test assuming dominant genetic model, the statistical power for the association between haplotype AGAAA of the SLCO1B1 gene and AUC0−t at 40 mg dose was calculated to be 0.21. In a 0.8 powered study, it is estimated that about five times the number of samples is needed to confirm the results. It is therefore critical to extend current findings to the analyses of large populations.
In conclusion, we characterized the LD structures and haplotypes of SLCO1B1, SLCO1B3 and ABCC2. The LD structures were different among three ethnic populations, but the mean olmesartan plasma concentration vs time profiles were same among them. These results may contribute to the framework of a future study on those transporters relevant to the variation in the pharmacokinetics of drugs. Indeed, the effects of associated haplotypes on the pharmacokinetics of olmesartan and the functional consequences warrant further study.
References
Laeis, P., Püchler, K., Kirch, W. The pharmacokinetic and metabolic profile of olmesartan medoxomil limits the risk of clinically relevant drug interaction. J. Hypertens. 19, S21–S32 (2001).
Nakagomi-Hagihara, R., Nakai, D., Kawai, K., Yoshigae, Y., Tokui, T., Abe, T. et al. OATP1B1, OATP1B3, and MRP2 are involved in hepatobiliary transport of olmesartan, a novel angiotensin II blocker. Drug Metab. Dispos. 34, 862–869 (2006).
Yamada, A., Maeda, K., Kamiyama, E., Sugiyama, D., Kondo, T., Shiroyanagi, Y. Multiple human isoforms of drug transporters contribute to the hepatic and renal transport of olmesartan, a selective antagonist of the angiotensin II AT1-receptor. Drug Metab. Dispos. 35, 2166–2176 (2007).
Tirona, R. G., Leake, B. F., Merino, G., Kim, R. B. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J. Biol. Chem. 276, 35669–35675 (2001).
Tirona, R. G., Leake, B. F., Wolkoff, A. W., Kim, R. B. Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation. J. Pharmacol. Exp. Ther. 304, 223–228 (2003).
Kameyama, Y., Yamashita, K., Kobayashi, K., Hosokawa, M., Chiba, K. Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet. Genomics 15, 513–522 (2005).
Iwai, M., Suzuki, H., Ieiri, I., Otsubo, K., Sugiyama, Y. Functional analysis of single nucleotide polymorphisms of hepatic organic anion transporter OATP1B1 (OATP-C). Pharmacogenetics 14, 749–757 (2004).
Nishizato, Y., Ieiri, I., Suzuki, H., Kimura, M., Kawabata, K., Hirota, T. et al. Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmacokinetics. Clin. Pharmacol. Ther. 73, 554–565 (2003).
Niemi, M., Backman, J. T., Kajosaari, L. I., Leathart, J. B., Neuvonen, M., Daly, A. K. et al. Polymorphic organic anion transporting polypeptide 1B1 is a major determinant of repaglinide pharmacokinetics. Clin. Pharmacol. Ther. 77, 468–478 (2005).
Niemi, M., Kivistö, K. T., Hofmann, U., Schwab, M., Eichelbaum, M., Fromm, M. F. Fexofenadine pharmacokinetics are associated with a polymorphism of the SLCO1B1 gene (encoding OATP1B1). Br. J. Clin. Pharmacol. 59, 602–604 (2005).
Letschert, K., Keppler, D., König, J. Mutations in the SLCO1B3 gene affecting the substrate specificity of the hepatocellular uptake transporter OATP1B3 (OATP8). Pharmacogenetics 14, 441–452 (2004).
Wada, M., Toh, S., Taniguchi, K., Nakamura, T., Uchiumi, T., Kohno, K. et al. Mutations in the canilicular multispecific organic anion transporter (cMOAT) gene, a novel ABC transporter, in patients with hyperbilirubinemia II/Dubin-Johnson syndrome. Hum. Mol. Genet. 7, 203–207 (1998).
Toh, S., Wada, M., Uchiumi, T., Inokuchi, A., Makino, Y., Horie, Y. et al. Genomic structure of the canalicular multispecific organic anion-transporter gene (MRP2/cMOAT) and mutations in the ATP-binding-cassette region in Dubin-Johnson syndrome. Am. J. Hum. Genet. 64, 739–746 (1999).
Keitel, V., Nies, A. T., Brom, M., Hummel-Eisenbeiss, J., Spring, H., Keppler, D. A common Dubin-Johnson syndrome mutation impairs protein maturation and transport activity of MRP2 (ABCC2). Am. J. Physiol. Gastrointest. Liver Physiol. 284, G165–G174 (2003).
Ito, S., Ieiri, I., Tanabe, M., Suzuki, A., Higuchi, S., Otsubo, K. Polymorphism of the ABC transporter genes, MDR1, MRP1 and MRP2/cMOAT, in healthy Japanese subjects. Pharmacogenetics 11, 175–184 (2001).
Hirouchi, M., Suzuki, H., Itoda, M., Ozawa, S., Sawada, J., Ieiri, I. et al. Characterization of the cellular localization, expression level, and function of SNP variants of MRP2/ABCC2. Pharm. Res. 21, 742–748 (2004).
Haenisch, S., Zimmermann, U., Dazert, E., Wruck, C. J., Dazert, P., Siegmund, W. et al. Influence of polymorphisms of ABCB1 and ABCC2 on mRNA and protein expression in normal and cancerous kidney cortex. Pharmacogenomics J. 7, 56–65 (2007).
Niemi, M., Arnold, K. A., Backman, J. T., Pasanen, M. K., Gödtel-Armbrust, U., Wojnowski, L. et al. Association of genetic polymorphism in ABCC2 with hepatic multidrug resistance-associated protein 2 expression and pravastatin pharmacokinetics. Pharmacogenet Genomics 16, 801–808 (2006).
Haiman, C. A., Stram, D. O., Pike, M. C., Kolonel, L. N., Burtt, N. P., Altshuler, D. et al. A comprehensive haplotype analysis of CYP19 and breast cancer risk: the Multiethnic Cohort. Hum. Mol. Genet. 12, 2679–2692 (2003).
Gabriel, S. B., Schaffner, S. F., Nguyen, H., Moore, J. M., Roy, J., Blumenstiel, B. et al. The structure of haplotype blocks in the human genome. Science 296, 2225–2229 (2002).
Daly, M. J., Rioux, J. D., Schaffner, S. F., Hudson, T. J., Lander, E. S. High-resolution haplotype structure in the human genome. Nat. Genet. 29, 229–232 (2001).
Goldstein, D. B., Tate, S. K., Sisodiya, S. M. Pharmacogenetics goes genomic. Nat. Rev. Genet. 4, 937–947 (2003).
International HapMap Consortium A haplotype map of the human genome. Nature 437, 1299–1320 (2005).
International HapMap Consortium A second generation human haplotype map of over 3.1 million SNPs. Nature 449, 851–861 (2007).
Horikawa, Y., Oda, N., Cox, N. J., Li, X., Orho-Melander, M., Hara, M. et al. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat. Genet. 26, 163–175 (2000).
Drysdale, C. M., McGraw, D. W., Stack, C. B., Stephens, J. C., Judson, R. S., Nandabalan, K. et al. Complex promoter and coding region beta 2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc. Natl Acad. Sci. USA 97, 10483–10488 (2000).
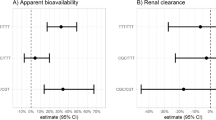
Rohatagi, S., Carrothers, T. J., Kshirsagar, S., Khariton, T., Lee, J., Salazar, D. Evaluation of population pharmacokinetics and exposure-response relationship with coadministration of amlodipine besylate and olmesartan medoxomil. J. Clin. Pharmacol. 48, 823–836 (2008).
Ewing, B., Hillier, L., Wendl, M., Green, P. Basecalling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 8, 175–185 (1998).
Ewing, B., Green, P. Basecalling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 8, 186–194 (1998).
Gordon, D., Abajian, C., Green, P. Consed: a graphical tool for sequence finishing. Genome Res. 8, 195–202 (1998).
Nickerson, D. A., Tobe, V. O., Taylor, S. L. polyphred: automating the detection and genotyping of single nucleotide substitutions using fluorescence-based resequencing. Nucleic Acids Res. 25, 2745–2751 (1997).
Wigginton, J. E., Cutler, D. J., Abecasis, G. R. A note on exact tests of Hardy-Weinberg equilibrium. Am. J. Hum. Genet. 76, 887–893 (2005).
Shibata, K., Ito, T., Kitamura, Y., Iwasaki, N., Tanaka, H., Kamatani, N. Simultaneous estimation of haplotype frequencies and quantitative trait parameters: applications to the test of association between phenotype and diplotype configuration. Genetics 168, 525–539 (2004).
R Development Core Team. R: A language and environment for statistical computing., R Foundation for Statistical Computing: Vienna, Austria) ISBN 3-900051-07-0 (2010). http://www.R-project.org/.
Cockcroft, D. W., Gault, M. H. Prediction of creatinine clearance from serum creatinine. Nephron 16, 31–41 (1976).
Benjamini, Y., Hocheberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. Royal Stat. Soc. Ser. B 57, 289 (1995).
Maeda, K., Ieiri, I., Yasuda, K., Fujino, A., Fujiwara, H., Otsubo, K. et al. Effects of organic anion transporting polypeptide 1B1 haplotype on pharmacokinetics of pravastatin, valsartan, and temocapril. Clin. Pharmacol. Ther. 79, 427–439 (2006).
Mwinyi, J., Johne, A., Bauer, S., Roots, I., Gerloff, T. Evidence for inverse effects of OATP-C (SLC21A6) 5 and 1b haplotypes on pravastatin kinetics. Clin. Pharmacol. Ther. 75, 415–421 (2004).
Smith, N. F., Marsh, S., Scott-Horton, T. J., Hamada, A., Mielke, S., Mross, K. et al. Variants in the SLCO1B3 gene: interethnic distribution and association with paclitaxel pharmacokinetics. Clin. Pharmacol. Ther. 81, 76–82 (2007).
Acknowledgements
We thank Ayako Mashimo and Reiko Iwakami for assistance with the genotyping and Kazuhito Shiosakai for encouraging discussions. This study was sponsored by Daiichi Sankyo Pharma Development.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Endo, S., Fukahori, A., Tokuhiro, S. et al. Association study of genetic polymorphisms of drug transporters, SLCO1B1, SLCO1B3 and ABCC2, in African-Americans, Hispanics and Caucasians and olmesartan exposure. J Hum Genet 57, 531–544 (2012). https://doi.org/10.1038/jhg.2012.63
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2012.63
Keywords
This article is cited by
-
Comments on: “Population Pharmacokinetic Modeling of Olmesartan, the Active Metabolite of Olmesartan Medoxomil, in Patients with Hypertension”
European Journal of Drug Metabolism and Pharmacokinetics (2017)