Abstract
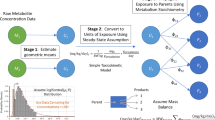
This study examines the use of physiologically based pharmacokinetic (PBPK) models for inferring exposure when the number of biomarker observations per individual is limited, as commonly occurs in population exposure surveys. The trade-off between sampling multiple biomarkers at a specific time versus fewer biomarkers at multiple time points was investigated, using a simulation-based approach based on a revised and updated chlorpyrifos PBPK model originally published. Two routes of exposure, oral and dermal, were studied as were varying levels of analytic measurement error. It is found that adding an additional biomarker at a given time point adds substantial additional information to the analysis, although not as much as the addition of another sampling time. Furthermore, the precision of the estimates of exposed dose scaled approximately with the analytic precision of the biomarker measurement. For acute exposure scenarios such as those considered here, the results of this study suggest that the number of biomarkers can be balanced against the number of sampling times to obtain the most efficient estimator after consideration of cost, intrusiveness, and other relevant factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Andersen M.E., Clewell III H.J., Gargas M.L., Smith F.A., and Reitz R.H. Physiologically based pharmacokinetics and the risk assessment process for methylene chloride. Toxicol Appl Pharmacol 1987: 87 (2): 185–205.
Barr D.B., Allen R., Olsson A.O., Bravo R., Caltabiano L.M., and Montesano A., et al. Concentrations of selective metabolites of organophosphorus pesticides in the United States population. Environ Res 2005: 99 (3): 314–326.
Barr D.B., Bravo R., Weerasekera G., Caltabiano L.M., Whitehead R.D., and Olsson A.O., et al. Concentrations of dialkyl phosphate metabolites of organophosphorous pesticides in the U.S. population. Environ Health Perspect 2004: 112 (2): 186–200.
Brown R.P., Delp M.D., Lindstedt S.L., Rhomberg L.R., and Beliles R.P. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 1997: 13: 407–484.
Clewell H.J., Gearhart J.M., Gentry P.R., Covington T.R., VanLandingham C.B., Crump K.S., and Shipp A.M. Evaluation of the uncertainty in an oral reference dose for methylmercury due to interindividual variability in pharmacokinetics. Risk Anal 1999: 19 (4): 547–558.
Dow AgroSciences. Chlorpyrifos –– North America 2007: http://www.dowagro.com/chlorp/na/about/.
Dow Chemical Co. Simusolv –– Modeling and Simulation Software. Dow Chemical Co., Midland, MI, 1990.
Egeghy P.P., Quackenboss J.J., Catlin S., and Ryan P.B. Determinants of temporal variability in NHEXAS-Maryland environmental concentrations, exposures, and biomarkers. J Expo Anal Environ Epidemiol 2005: 15 (5): 388–397.
Furlong C.E., Richter R.J., Seidel L., Costa G., and Motulsky A.G. Spectrophotometric assays for the enzymatic hydrolysis of the active metabolites of chlorpyrifos and parathion by plasma paraoxonase/arylesterase. Anal Biochem 1989: 180: 242–247.
Garfitt S.J., Jones K., Mason H.J., and Cocker J. Exposure to the organophosphate diazinon: data from a human volunteer study with oral and dermal doses. Toxicol Lett 2002: 134 (1–3): 105–113.
Gearhart J.M., Jepson G.W., Clewell III H.J., Andersen M.E., and Conolly R.B. Physiologically based pharmacokinetic and pharmacodynamic model for the inhibition of acetylcholinesterase by diisopropylfluorophosphate. Toxicol Appl Pharmacol 1990: 106: 295–310.
Gelman A., Bois F.Y., and Jiang J. Physiological pharmacokinetic analysis using population modeling and informative prior distributions. J Am Stat Assoc 1996: 91: 1400–1412.
Georgopoulos P., Roy A., and Gallo M.A. Reconstruction of short-term multi-route exposure to volatile organic compounds using physiologically based pharmacokinetic models. J Expo Anal Environ Epidemiol 1994: 4 (3): 309–328.
Griffin P., Mason H., Heywood K., and Cocker J. Oral and dermal absorption of chlorpyrifos: a human volunteer study. Occup Environ Med 1999: 56: 10–13.
Hayes A.W. Principles and Methods of Toxicology, 4th edn. Raven Press, New York, 2001.
Henderson R.F. Strategies for the use of biomarkers of exposure. Toxicol Lett 1995: 82/83: 379–383.
Klaassen C.D. Casarett and Doull's Toxicology: the Basic Science of Poisons, 6th edn. McGraw-Hill, New York, 2001.
McKone T.E. Linking a PBPK model for chloroform with measured breath concentrations in showers: implications for dermal exposure models. J Expo Anal Environ Epidemiol 1993: 3: 339–365.
Morgan M.K., Sheldon L.S., Croghan C.W., Jones P.A., Robertson G.L., and Chuang J.C., et al. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J Expo Anal Environ Epidemiol 2005: 15: 297–309.
Nash R.G., Kearney P.C., Maitlen J.C., Sell C.R., and Fertig S.N. Agricultural applicators exposure to 2,4-dichlorophenoxyacetic acid. ACS Symp Ser 1982: 182: 119–132.
National Research Council. Human Biomonitoring for Environmental Chemicals. The National Academies Press, Washington DC, 2006.
Needham L.L., Ashley D.L., and Patterson D.G. Case studies of the use of biomarkers to assess exposures. Toxicol Lett 1995: 82/83: 373–378.
Nolan R.L., Rick D.L., Freshour N.L., and Saunders J.H. Chlorpyrifos: pharmacokinetics in human volunteers. Toxicol Appl Pharmacol 1984: 73: 8–15.
Pang Y., MacIntosh D.L., Camann D.E., and Ryan P.B. Analysis of Aggregate exposure to chlorpyrifos in the NHEXAS-Maryland investigation. Environ Health Persp 2002: 110: 235–240.
Ramsey J.C., and Andersen M.E. A physiologically based description of the inhalation pharmacokinetics of styrene monomer in rats and humans. Toxicol Appl Pharmacol 1984: 73: 159–175.
Reddy M., Yang R.S., Andersen M.E., and Clewell H.J. Physiologically Based Pharmacokinetic Modeling: Science and Applications. John Wiley & Sons Inc, Hoboken, NJ, 2005.
Rigas M.L., Okino M.S., and Quackenboss J.J. Use of a pharmacokinetic model to assess chlorpyrifosexposure and dose in children based on urinary biomarker measurements. Toxicol Sci 2001: 61: 374–381.
Roy A., and Georgopoulos P.G. Reconstructing week-long exposures to volatile organic compounds using physiologically based pharmacokinetic models. J Expo Anal Environ Epidemiol 1998: 8 (3): 407–422.
Sams C., Cocker J., and Lennard M.S. Biotransformation of chlorpyrifos and diazinon by human liver microsomes and recombinant human cytochrome P450s (CYP). Xenobiotica 2004: 34: 861–873.
Sohn M.D., McKone T.E., and Blancato J.N. Reconstructing population exposures from dose biomarkers: inhalation of trichloroethylene (TCE) as a case study. J Expo Anal Environ Epidemiol 2004: 14: 204–213.
Sultatos L.G. Mammalian toxicology of organophosphorus pesticides. J Toxicol Environ Health 1994: 43: 271–289.
Tan Y.M., Liao K.H., and Clewell H.J. Reverse dosimetry: interpreting trihalomethanes biomonitoring data using physiologically based pharmacokinetic modeling. J Expo Sci Environ Epidemiol 2007: 17: 591–603.
Tan Y.M., Liao K.H., Conolly R.B., Blount B.C., Mason A.M., and Clewell H.J. Use of a physiologically based pharmacokinetic model to identify exposures consistent with human biomonitoring data for chloroform. J Toxicol Environ Health A 2006: 69 (18): 1727–1756.
Timchalk C., Kousba A., and Poet T.S. Monte Carlo analysis of the human chlorpyrifos-oxonase (PON1) polymorphism using a physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model. Toxicol Lett 2002a: 135: 51–59.
Timchalk C., Nolan R.J., Mendrala D.A., Dittenber D.A., Brzak K.A., and Mattson J.L. A physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model for the organophosphate insecticide chlorpyrifos in rats and humans. Toxicol Sci 2002b: 66: 34–53.
USDHHS. Toxicological Profile for Chlorpyrifos. US Dept. of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1997.
Wakefield J. The Bayesian analysis of population pharmacokinetic models. J Am Stat Assoc 1996: 91 (433): 62–75.
Wallace L., Pellizari E., and Gordon S. A linear model relating breath concentrations to environmental exposures: application to a chamber study of four volunteers exposed to volatile organic chemicals. J Expo Anal Environ Epidemiol 1993: 3: 75–102.
Wilson N.K., Chuang J.C., Lyu C., Menton R., and Morgan M.K. Aggregate exposures of nine preschool children to persistent organic pollutants at day care and at home. J Expo Anal Environ Epidemiol 2003: 13: 187–202.
Acknowledgements
We thank Dr. Charles Timchalk for generously providing his SIMUSOLV® (a registered trademark of the Dow Chemical Co., Midland, MI) code and output files, and Dr. Joel Mattsson and the Dow Chemical Company for provision of the human volunteer CPF exposure data described in Nolan et al. (1984). We also thank Dr. Sydney Gordon of Battelle, and Mr. Andy Clayton, Dr. Cary Eaton, Ms. Amy Etheridge, Dr. Timothy Fennell, Dr. James Raymer, Ms. Carol Sloan and Dr. Roy Whitmore of RTI for their review and administrative support of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclaimer
The United States Environmental Protection Agency through its Office of Research and Development funded and collaborated in the research described here under contract EP-D04-068 to Battelle with a subcontract to the RTI International. It has been subjected to Agency review and approved for publication.
Appendix
Appendix
PBPK model equations, both ordinary differential and algebraic, that are different from Timchalk et al. (2002b), as well as the definitions of terms used in the model, are presented here:
Liver Compartment CPF Model

Liver Compartment CPF-oxon Model

Blood Compartment CPF-oxon Model

One-Compartment TCP

One-Compartment DEP

One-Compartment DETP

Definition of terms used in the model
d=change (unitless)
AH=amount (μmol) of CPF in the liver
t=time (h)
QH=blood flow (h) to the liver
CAHf=free CPF concentration (μmol/l) in arterial blood entering the liver
CVHf=free CPF concentration (μmol/l) in venous blood leaving the liver
dOral/dH=rate of uptake (μmol/h) into the liver compartment from the GI tract
CH=CPF concentration (μmol/l) in the liver
Vmax1=maximum rate (μmol/h) for metabolism (CPF to CPF-oxon)
Km1=Michaelis–Menten constant (μmol/l) for metabolism (CPF to CPF-oxon)
Vmax2=maximum rate (μmol/h) for metabolism (CPF to TCP; CPF to DETP)
Km2=Michaelis–Menten constant (μmol/l) for metabolism (CPF to TCP; CPF to DETP)
AHo=amount (μmol) of CPF-oxon in liver
Vmax3=maximum rate (μmol/h) for metabolism (CPF-oxon to TCP (liver), CPF-oxon to DEP (liver))
Km3=Michaelis–Menten constant (μmol/l) for metabolism (CPF-oxon to TCP (liver); CPF-oxon to DEP (liver))
CAHof=free CPF-oxon concentration (μmol/l) in arterial blood entering the liver
CVHof=free CPF-oxon concentration (μmol/l) in venous blood leaving the liver
ABL=amount (μmol) of CPF-oxon in mixed blood compartment
QC=cardiac output (l/h)
CVmof=free CPF-oxon concentration (μmol/l) in mixed blood compartment
CVeof=free CPF-oxon concentration (μmol/l) in venous blood
Vmax4=maximum rate (μmol/h) for metabolism (CPF-oxon to TCP (blood); CPF-oxon to DEP (blood))
Km4=Michaelis–Menten constant (μmol/l) for metabolism (CPF-oxon to TCP (blood); CPF-oxon to DEP (blood))
ATCP=amount (μmol) of TCP
KeTCP=urinary elimination rate (h−1) for TCP
VdTCP=volume of distribution (l) for TCP
cbTCP=TCP concentration (μmol/l) in blood
ADEP=amount (μmol) of DEP
KeDEP=urinary elimination rate (h−1) for DEP
VdDEP=volume of distribution (l) for DEP
cbDEP=DEP concentration (μmol/l) in blood
ADETP=amount (μmol) of DETP
KeDETP=urinary elimination rate (h−1) for DETP
VdDETP=volume of distribution (l) for DETP
cbDETP=DETP concentration (μmol/l) in blood
Rights and permissions
About this article
Cite this article
Mosquin, P., Licata, A., Liu, B. et al. Reconstructing exposures from small samples using physiologically based pharmacokinetic models and multiple biomarkers. J Expo Sci Environ Epidemiol 19, 284–297 (2009). https://doi.org/10.1038/jes.2008.17
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jes.2008.17
Keywords
This article is cited by
-
Inter-individual variation in chlorpyrifos toxicokinetics characterized by physiologically based kinetic (PBK) and Monte Carlo simulation comparing human liver microsome and Supersome™ cytochromes P450 (CYP)-specific kinetic data as model input
Archives of Toxicology (2022)
-
Prediction of dose-dependent in vivo acetylcholinesterase inhibition by profenofos in rats and humans using physiologically based kinetic (PBK) modeling-facilitated reverse dosimetry
Archives of Toxicology (2021)