Abstract
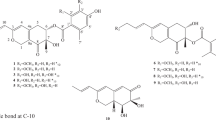
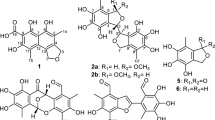
Four new xanthonoids, versicones E–H (1–4), and a biogenetically related new derivative arugosin K (5), were isolated from a culture extract of the mangrove-derived fungus Aspergillus versicolor HDN11-84. Their structures were elucidated on the basis of extensive NMR spectroscopic data analysis. Versicone E (1) represents the first example of naturally occurring xanthonoids containing a 2-butenamide moiety. Among them, 5 showed the best cytotoxicity against Hela cell line with an IC50 value of 9.2 μm.
Similar content being viewed by others
Introduction
Xanthonoids, a family of polyketides with a polyphenolic dibenzo-γ-pyrone core, are widely distributed in plants, fungi and lichens,1, 2, 3, 4, 5, 6 and show various biological activities partly depending on the variation of substituents.7 Prenylation is an important post-modification process during the biosynthesis of xanthones,1, 8 through which, diverse prenylated xanthones are provided with a range of enhanced bioactivities including antimicrobial, antioxidant, cytotoxic, antiviral, antiallergic, anti-inflammatory, antimalarial and antihyperglycemic effects.7, 9, 10
Xanthones from lichens and fungi are mainly derived from acetate units.7, 11, 12 Among naturally occurring xanthones, prenylated derivatives make up an important subgroup.11, 13 Typically, the five-carbon dimethylallyl moiety is directly attached to the aromatic rings of xanthonoids via the C–C bond. Some rare instances are the structures where the prenyl units are linked via oxygen bridges, for which only a few cases have been reported, such as variecoxanthon A14 and versicones A–D.15 The O-prenylation of xanthones was reported to be catalyzed by XptB under the presence of dimethylallyl diphosphate.1
During our searching for novel bioactive secondary metabolites from microorganisms derived from various habitats,16, 17, 18 a mangrove rhizosphere-derived Aspergillus versicolor strain HDN11-84, was selected based on the characteristic HPLC–UV profile of the ethyl acetate (EtOAc) extract. Guided by the UV characteristics, five new polyketides, including four xanthonoids versicones E–H (1–4) and a biogenetically related derivative arugosin K (5) (Figure 1) were isolated from the culture extract, together with four known ones versicones A, B, D (6–8) and variecoxanthone A (9). The cytotoxicity of new compounds was tested against K562, HL-60, Hela, HCT-116 and NB4 cell lines. Compound 5 showed the best activity against Hela cell line with an IC50 (half maximal inhibitory concentration) value of 9.2 μm.
Experimental procedure
General
UV spectra were recorded on a Beckman DU 640 spectrophotometer (Beckman, Shanghai, China). IR spectra were recorded on a Nicolet NEXUS 470 spectrophotometer (Thermo Scientific, Beijing, China) in KBr disks. NMR spectra were recorded on JEOL JUM-ECP 600 (JEOL, Beijing, China) and Agilent 500 MHz DD2 spectrometers (Agilent, Beijing, China) using tetramethylsilane as an internal standard, and the chemical shifts were recorded as δ values. HRESIMS and ESIMS data were obtained on a Thermo Scientific LTQ Orbitrap XL mass spectrometer. TLC and column chromatography were performed either on plates precoated with silica gel GF254 (10–40 μm) or over silica gel (200–300 mesh, Qingdao Marine Chemical Factory, Qingdao, China). Size-exclusion chromatography was performed using the Sephadex LH-20 (GE Healthcare, Bio-Sinences, Piscataway, NJ, USA). Semi-preparative HPLC was performed using an octadecylsilyl (ODS) column (YMC-Pack ODS-A, 10 × 250 mm, 5 μm).
Fungal resource
The fungus A. versicolor HDN11-84 was isolated from rhizosphere soil of mangrove plant Thespesia populnea, collected in Guangxi Province, China. The isolate was identified by ITS-rDNA sequence analysis. The ITS1-5.8S-ITS2 rDNA sequence of the strain HDN11-84 was submitted to GenBank with the accession number KU950433. The voucher specimen has been deposited in our laboratory at −80 °C. The working strain was prepared on potato dextrose agar in slants and stored at 4 °C.
Fermentation and extraction
A small spoon of spores growing on potato dextrose agar slant was inoculated into a 500 ml Erlenmeyer flask containing 150 ml culture medium comprising of 20.0% potato broth, 1% maltose, 0.5% peptone, 2.0% glucose, and 2.0% mannitol, 0.3% yeast extract (in sea water) cultured at 28 °C for 2 days on a rotary shaker at 160 r.p.m. Then 3 ml seed culture was inoculated into 1000 ml medium containing 300 ml of the above culture medium (pH=5.7). The fungus was grown under the static condition in 200 Erlenmeyer flasks (1 L). After 3 weeks of fermentation (pH=7.3), the mycelium and broth were separated by filtration and the broth extracted with equal volumes of EtOAc twice. The mycelium was macerated and extracted with acetone, which was evaporated in vacuum and then extracted with EtOAc. All of the EtOAc extracts were combined to give a crude gum (25 g).
Purification
The EtOAc extract (25 g) was subjected to ODS over a silica gel column using a gradient elution with MeOH/H2O to give five fractions (fraction 5.1–5.5). Fraction 5.5 was subjected to Sephadex LH-20 chromatography (MeOH:CH2Cl2=1:1) to afford five fractions (fraction 5.5.1–5.5.5). The fraction 5.5.5 was further purified by HPLC (90% MeOH/H2O, 3 ml min−1) to obtain compounds 3 (tR 9.3 min; 13.5 mg) and 2 (tR 14.6 min; 10.5 mg). Fraction 5.5.4 was subjected to Sephadex LH-20 chromatography (MeOH:CH2Cl2=1:1) to yield four fractions (fraction 5.5.4.1–5.5.4.4). Fraction 5.5.4.1 was subjected to semi-preparative HPLC (65% MeOH/H2O, 3 ml min−1) and further purified by HPLC (80% MeOH/H2O, 3 ml min−1) to afford compounds 1 (tR 12.3 min; 13.4 mg) and 4 (tR 17.8 min; 3.6 mg), as well as 5 (65% MeCN/H2O, 3 ml min−1; tR 20.8 min; 4.6 mg). The MS and NMR spectra of 1-5 are in Supplementary Information.
Versicone E (1)
Yellow oil; UV (MeOH) λmax (log ɛ) 220 (1.2), 263 (0.6), 332 (0.3) nm; IR: 3342, 2931, 1675, 1645, 1616, 1475, 1267, 1209, 1097, 1014 cm−1; for 1H and 13C NMR data, see Tables 1 and 2; HRESIMS m/z 384.1451 [M+H]+ (calcd for C21H22O6N, 384.1442).
Versicone F (2)
Yellow amorphous powder; UV (MeOH) λmax (log ɛ) 220 (2.0), 342 (0.6) nm; IR: 2919, 2850, 1648, 1463, 1425, 1276, 1070 cm−1; for 1H and 13C NMR data, see Tables 1 and 2; HRESIMS m/z 385.1655 [M+H]+ (calcd for C22H25O6, 385.1646).
Versicone G (3)
Brownish yellow oil; UV (MeOH) λmax (log ɛ) 221 (2.0), 343 (0.3) nm; IR: 2931, 2858, 1735, 1658, 1540, 1483, 1459, 1429, 1276, 1247, 1072, 1029 cm−1; for 1H and 13C NMR data, see Tables 1 and 2; HRESIMS m/z 427.1754 [M+H]+ (calcd for C24H27O7, 427.1751).
Versicone H (4)
Yellow oil; UV (MeOH) λmax (log ɛ) 218 (1.3), 349 (0.3) nm; IR: 3473, 2942, 2902, 1643, 1612, 1486, 1465, 1417, 1280, 1068, 1018 cm−1; for 1H and 13C NMR data, see Tables 1 and 2; HRESIMS m/z 353.0998 [M+Na]+ (calcd for C18H18O6Na, 353.0996).
Arugosin K (5)
Yellow amorphous powder; UV (MeOH) λmax (log ɛ) 258 (1.2) nm; IR: 2973, 2929, 1743, 1610, 1475, 1457, 1228, 1120 cm−1; for 1H and 13C NMR data, see Table 3; HRESIMS m/z 481.2227 [M+H]+ (calcd for C28H33O7, 481.2221).
Cytotoxicity assay
Cytotoxic activities of 1 –5 were evaluated using K562, Hela, NB4, HL-60 (using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method) and HCT-116 (using the sulforhodamine B (SRB) method).19, 20 Doxorubicin hydrochloride was used as positive control (IC50 values 0.30, 0.60, 0.40, 0.02 and 0.20 μm, respectively). Compounds 3 and 5 exhibited cytotoxicity against Hela, HL-60 and NB4 cell lines with IC50 values ranging from 9.2 to 21.7 μm.
Results and discussion
Versicone E (1) was obtained as yellow oil. Its formula C21H21O6N was determined by the protonated peak at HRESIMS peak at m/z 384.1451. The 1D NMR data (Tables 1 and 2) showed 21 carbon signals classified by DEPT and HSQC spectra as three methyls with one methoxy, two methylenes, five aromatic/olefinic methines and 11 non-protonated carbons including two carbonyls (δC 177.9 and 170.0). The 1H and 13C NMR data of 1 suggested a xanthone core skeleton, which was further indicated by the COSY (H-2/H-3) and HMBC correlations (Figure 2). Comparing with the reported 7-dihydroxy-6-methyl-8-hydroxymethylxanthone,1 the major differences belonged to the signals of the substituent groups at C-1 (δC 160.4) and C-7 (δC 152.4). The substituent of C-1 was deduced to be a methoxyl based on the HMBC correlation between H3-13 (δH 3.91) and C-1, as well as the chemical shifts of C-13 (δC 56.6). The COSY (H2-1′/H-2′) and HMBC correlations from H2-1′ (δH 4.58) to C-7/C-2′ and from H3-5′ (δH 1.76) to C-3′ (δC 134.2)/C-2′ (δC 130.0)/C-4′ (δC 170.0) suggested the existence of a 2-butenamide fragment, which was located on C-7. The enhancement of H2-1′ when irradiated H3-5′ in the 1D-NOE (Supplementary Figure S6) experiment indicated the E geometry of the double bond in 2-butenamide. Compound 1 was named as versicone E.
Versicone F (2) was obtained as yellow amorphous powder with a molecular formula C22H24O6 suggested by the protonated HRESIMS peak at m/z 385.1655. The 1D NMR data (Tables 1 and 2) showed 22 carbon signals including five methyls with two oxygenated, two methylenes, four methines and 11 non-protonated carbons. The 1H and 13C NMR spectra of 2 showed similar data to those of variecoxanthone A (8).14 The difference was the appearance of an additional methoxyl group CH3-14 (δH 3.97, δC 61.6) that was located on C-2 (δC 148.5) by the HMBC correlation from H3-14 to C-2.
Versicones G and H (3 and 4) were obtained with molecular formula C24H26O7 and C18H18O6, respectively. Compound 3 showed similar NMR data to versicone B (7),15 and their difference was the replacement of the OH group in 7 by an acetate-substituted hydroxymethyl in 3 supported by the HMBC correlations of H2-11 (δH 5.78) to C-1″ (δC 170.9) and H3-2″ (δH 2.06) to C-1″. Although in compound 4, the substituent of C-7 (δC 153.5) was changed to a methoxy group compared with versicone B (7), which was deduced by the HMBC correlation between H3-15 (δH 3.76) and C-7.
Arugosin K (5) was obtained as yellow amorphous powder. Its molecular formula was determined as C28H32O7 evidenced by the HRESIMS protonated ion peak at m/z 481.2227. The 1H and 13C NMR data (Table 3) of 5 were similar to those of arugosin E,21 indicating that they shared the same structural skeleton. The differences of two compounds were the substituents at C-3 (δC 151.6) and C-7 (δC 132.0). In arugosin E, the substituent at C-7 was a formaldehyde, whereas in 5, it changed to a methylene, which was further connected to an acetate-substituted hydroxymethyl as evidenced from the HMBC correlations of H2-19 (δH 5.11) to C-7 and C-1″ (δC 170.4), H3-2″ (δH 1.75) to C-1″. The HMBC correlation of H3-21 (δH 3.69) to C-3 indicated the substituent of C-3 was a methoxy group, instead of a hydroxyl group in arugosin E.
The in vitro cytotoxic activity of new compounds (1–5) was tested on five human cancer cell lines, K562 (myelogenous leukemia), Hela (human cervical cancer), NB4 (acute promyelocytic leukemia), HL-60 (human leukemia) and HCT-116 (human colon cancer). Compounds 3 and 5 showed cytotoxicity against Hela, HL-60 and NB4 cell lines with IC50 values ranging from 9.2 to 21.7 μm (Table 4).
In conclusion, nine polyketides including five new compounds (1–5) and four known ones (6–9) were discovered from the mangrove-derived fungus A. versicolor HDN11-84. Among them, versicone E (1) is the first example of natural xanthanonoids containing 2-butenamide moiety.
References
Pockrandt, D., Ludwig, L., Fan, A., Koenig, G. M. & Li, S. M. New insights into the biosynthesis of prenylated xanthones: Xptb from Aspergillus nidulans catalyses an O-prenylation of xanthones. Chembiochem. 13, 2764–2771 (2012).
Peres, V. & Nagem, T. J. Trioxygenated naturally occurring xanthones. Phytochemistry 44, 191–214 (1997).
Peres, V. & Nagem, T. J. Naturally occurring pentaoxygenated, hexaoxygenated and dimeric xanthones: a literature survey. Quim. Nova. 20, 388–397 (1997).
Peres, V., Nagem, T. J. & de Oliveira, F. F. Tetraoxygenated naturally occurring xanthones. Phytochemistry 55, 683–710 (2000).
Vieira, L. M. M. & Kijjoa, A. Naturally-occurring xanthones: recent developments. Curr. Med. Chem. 12, 2413–2446 (2005).
Bennett, G. J. & Lee, H. H. Xanthones from Guttiferae. Phytochemistry 8, 967–998 (1989).
El-Seedi, H. R. et al. Recent insights into the biosynthesis and biological activities of natural xanthones. Curr. Med. Chem. 17, 854–901 (2010).
Sanchez, J. F. et al. Genome-based deletion analysis reveals the prenyl xanthone biosynthesis pathway in Aspergillus nidulans. J. Am. Chem. Soc. 133, 4010–4017 (2011).
Pedraza, C. J., Cardenas, R. N., Orozco, I. M. & Perez, R. J. M. Medicinal properties of mangosteen (Garcinia mangostana. Food Chem. Toxicol. 46, 3227–3239 (2008).
Laphookhieo, S., Syers, J. K., Kiattansakul, R. & Chantrapromma, K. Cytotoxic and antimalarial prenylated xanthones from Cratoxylum cochinchinense. Chem. Pharm. Bull. 54, 745–747 (2006).
El-Seedi, H. R. et al. Naturally occurring xanthones; latest investigations: isolation, structure elucidation and chemosystematic significance. Curr. Med. Chem. 16, 2581–2626 (2009).
Masters, K & Bräse, S. Xanthones from fungi, lichens, and bacteria: the natural products and their synthesis. Chem. Rev. 112, 3717–3776 (2012).
Pinto, M. M. M. & Castanheiro, R. A. P. Synthesis of prenylated xanthones: an overview. Curr. Org. Chem. 13, 1215–1240 (2009).
Chexal, K. K., Holker, J. S. E., Simpson, T. J., Young, K. & Robinson, R. The biosynthesis of fungal metabolites. Part VI structure of variecoxanthones A, B, and C, metabolites of Aspergiiius variecolor; conversion of variecoxanthone A into (±)-De-C-prenylepishamixanthone. J. Chem. Soc. Perkin Trans. 1 6, 543–548 (1975).
Wang, J. F. et al. Antifungal new oxepine-containing alkaloids and xanthones from the deep-sea-derived fungus Aspergillus versicolor SCSIO 05879. J. Agric. Food Chem. 64, 2910–2916 (2016).
Du, L. et al. Unprecedented citrinin trimer tricitinol B functions as a novel topoisomerase IIα inhibitor. J. Med. Chem. 54, 5796–5810 (2011).
Li, L. Y., Li, D. H., Luan, Y. P., Gu, Q. Q. & Zhu, T. J. Cytotoxic metabolites from the antarctic psychrophilic fungus Oidiodendron truncatum. J. Nat. Prod. 75, 920–927 (2012).
Du, L. et al. Aspergiolides C and D: spirocyclic aromatic polyketides with potent protein kinase c-Met inhibitory effects. Chem. Eur. J. 17, 1319–1326 (2011).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 65, 55–63 (1983).
Skehan, P. et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 82, 1107–1112 (1990).
Nobuo, K., Koohei, N., Shoichi, N. & Ken-ichi, K. Studies on fungal products. Part 15. Isolation and structure determination of arugosin E from Aspergillus silvaticus and cycloisoemericellin from Emericella striata. J. Chem. Soc. Perkin Trans. 1 4, 907–911 (1988).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21372208), the Shandong Provincial Natural Science Fund for Distinguished Young Scholars (JQ201422), the Program for New Century Excellent Talents in University (NCET-12-0499) and the NSFC-Shandong Joint Fund for Marine Science Research Centers (U1406402).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Li, F., Guo, W., Che, Q. et al. Versicones E–H and arugosin K produced by the mangrove-derived fungus Aspergillus versicolor HDN11-84. J Antibiot 70, 174–178 (2017). https://doi.org/10.1038/ja.2016.95
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2016.95
This article is cited by
-
Asperitaconic acids A–C, antibacterial itaconic acid derivatives produced by a marine-derived fungus of the genus Aspergillus
The Journal of Antibiotics (2018)