Abstract
We synthesized 7(S)-7-deoxy-7-arylthiolincomycin derivatives possessing a heterocyclic ring at the C-7 position via sulfur atom by either Mitsunobu reaction of 2,3,4-tris-O-(trimethylsiliyl)lincomycin or SN2 reaction of 7-O-methanesulfonyl-2,3,4-tri-O-trimethylsiliyllincomycin. As a result, 7(S)-7-deoxy-7-arylthiolincomycin derivatives 16, 21 and 27 exhibited antibacterial activities against respiratory infection-related Gram-positive bacteria with erm gene, although clindamycin did not have any activities against those pathogens. Furthermore, 7(S)-configuration of lincomycin derivatives was found to be necessary for enhancing antibacterial activities from the comparison results of configurations of 16 (S-configuration) and 30 (R-configuration) at the 7-position.
Similar content being viewed by others
Introduction
Macrolide antibiotics possess broad spectrum of antibacterial activity against Gram-positive bacteria (Streptococcus pneumoniae, Streptococcus pyogenes, etc.), Haemophilus influenzae, Moraxella catarrhalis, Mycoplasma pneumoniae and Neisseria gonorrhoeae and also have a safety profile as oral drugs. Therefore, macrolide antibiotics have been used as chemotherapeutic agents in clinical sites over many years. Resistant bacteria, however, have markedly increased recently,1, 2, 3 and this phenomenon has caused serious problems in the treatment of bacterial respiratory infections.
Macrolide antibiotics inhibit bacterial protein synthesis. Macrolide antibiotics have potent antibacterial activities through inhibiting elongation of an amino-acid sequence by binding to 23S ribosomal RNA.4, 5, 6 The mechanisms of action for bacteria to gain resistance are manifold, but in general, these can be characterized as involving drug efflux, alterations in the drug target site or drug inactivation. Resistant mechanisms in macrolides have diversified recently. Notably, there are major bacterial strains in clinical site, with point mutation in the drug target site,7, 8 with methylase produced by erm gene and with efflux pump produced by mef gene.
The methylase of bacteria inhibits macrolide binding to 23S ribosomal RNA through either methylation or dimethylation at the N-6 position of the adenine residue (A2058Ec).7, 9, 10 On the other hand, the efflux pump of bacteria is able to transport a variety of compounds, thus conferring resistance to a broad range of antibiotics.
Clarithromycin11 and azithromycin12 (Figure 1) are not effective enough against resistant bacteria (S. pneumoniae and S. pyogenes) with erm gene and influenced by S. pneumoniae with mef gene. On the other hand, telithromycin (TEL),13 which was launched as the first ketolide antibiotic, is effective against resistant bacteria with erm and mef genes. X-ray crystallographic6, 14 and footprinting analysis15, 16, 17 indicated that TEL would be capable to bind to not only domain V (A2058Ec, A2059Ec) of 23S rRNA but also to domain II (A752). TEL, however, has possibility to cause serious liver damage18, 19 and loss of consciousness,20, 21 and it is scarcely used in Japan. No oral antibiotic, which is effective against the resistant bacteria with erm and mef genes, with no problems in terms of safety or taste, has been launched so far.
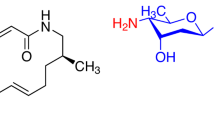
Lincomycin (LCM)22, 23, 24, 25 was isolated as a secondary metabolite from the fermentation broth of Streptomyces lincolnensis. Clindamycin (CLDM)26 was synthesized by chemical modification of LCM (Figure 1) and exhibited improved antimicrobial activities and pharmacokinetics compared with LCM. But they are not effective against resistant bacteria (S. pneumoniae and S. pyogenes) with erm gene (Table 1).
Chemical modifications at the C-7 positions of LCM (7-dehydrolincomycin (7-ketolincomycin),27 7-deoxylincomycin,28 lincomycin-7-acylate29 and lincomycin-7-carbonate,29 7(R)-7-azido-7-deoxylincomycin,25 7(R)-7-amino-7-deoxylincomycin,25 7(R)-7-cyano-7-deoxylincomycin,25 7(R)-7-deoxy-7-thiolincomycin,30 7(S)-7-deoxy-7-thiolincomycin,30 7(S)-7-bromo-7-deoxylincomycin,26 7(S)-7-deoxy-7-iodolincomycin,26 7(R)-7-chloro-7-deoxylincomycin,26 and 7-epilincomycin (7(S)-lincomycin),26 7-deoxy-7-methyllincomycin31 and so on) have been investigated so far. In these compounds, 7(S)-7-bromo-7-deoxylincomycin and 7(S)-7-deoxy-7-iodolincomycin exhibited enhanced antibacterial activities in the same order of magnitude as CLDM, and 7(R)-7-azido-7-deoxylincomycin had the same antibacterial activities as LCM.
In the case of possessing a chlorine atom at the 7-position, CLDM (7(S)-Cl) had stronger antibacterial activities than 7-epiclindamycin (7(R)-Cl). 7-Epilincomycin (7(S)-lincomycin) had one-half of the potency of the LCM possessing 7(R)-configuration (7(S)-OH<7(R)-OH).
7(R)-7-O-methyllincomycin, of which 7-hydrolyl group was protected, showed improved potency, whereas 7(S)-7-O-methyllincomycin had stronger activities of 3.5 times the response of LCM against Sarcina lutea (7(S)-OMe>7(R)-OMe).32, 33 Unfortunately, both larger alkoxy groups and substituted alkoxy groups resulted in weaker antibacterial activities than those of LCM.
On the other hand, both 7(S)-7-deoxy-7-thiolincomycin and 7(R)-7-deoxy-7-thiolincomycin showed only 10% activity as compared with LCM. 7(S)-7-alkylthio-7-deoxylincomycin and 7(S)-7-substituted alkylthio-7-deoxylincomycin34, 35, 36, 37, 38 were more active than LCM against Gram-positive or Gram-negative organisms, and 7(R)-7-deoxy-7-imidazol-2-yl-thiolincomycin, which had been reported by Sztaricskai et al.,39 retained the same order of magnitude of antibacterial activities against tested organisms as those of LCM. Antibacterial activities were affected by both configuration and a structure of a substituent at the 7-position. 7(S)-7-deoxy-7-phenylthiolincomycin was reported by Bannister et al.38 but no antibacterial activity of this compound was reported.
Thus we were interested in derivatives with a heterocyclic ring introduced via sulfur atom at the C-7 position with 7(S)-configuration. According to the above publications, LCM derivatives exhibited much stronger in vitro activities compared with LCM, but no LCM derivatives were effective against resistant bacteria with erm gene.
LCM and CLDM inhibited bacterial protein synthesis similar to macrolide antibiotics. The structures of lincomycin derivatives are different from those of macrolide antibiotics, but X-ray crystallographic analysis indicated that their binding sites to rRNA are closely located in a neighboring area.4, 6 According to the report,4, 6 there were several major interactions by hydrogen bonding between the peptidyl transferase cavities (A2058Ec, A2059Ec and G2520Ec) and hydroxyl groups at the sugar portion of CLDM. These data suggest that it is difficult for us to improve antibacterial activity by chemical modification at the sugar moiety. In fact, 2-deoxylincomycin40 has been reported to show only 1% activity compared with LCM.
As we previously described, TEL has a risk of causing serious side effects18, 19, 20, 21 and it is rarely used in Japan. Furthermore, its production cost seems to be relatively high owing to its complicated structure. On the other hand, CLDM possessing a simple structure has similar antibacterial activity against susceptible strains such as clarithromycin and exhibits acceptable oral absorption in animals and humans. However, CLDM is not effective enough against resistant bacteria of S. pneumoniae or S. pyogenes with erm gene. So a novel oral lincomycin analog, which is effective against resistant bacteria with erm gene and/or mef gene and does not have any problems in safety, taste or pharmacokinetics, is strongly desired for treatment of respiratory infections in clinical sites.
On the other hand, 7(S)-7-azido-7-deoxylincomycin, CLDM, 7(S)-7-bromo-7-deoxylincomycin and 7(S)-7-alkylthio-7-deoxylincomycin had the same or stronger antibacterial activity compared with LCM. According to the relationships between configuration and a substituent (7(S)-Cl>7(R)-Cl and 7(S)-OMe>7(R)-OMe), enhancement of activities in 7(S)-configuration might require a hydrophobic substituent such as a chlorine atom or a methyl group, not as OH or SH (7(S)-OH<7(R)-OH (LCM), 7(S)-SH≈7(R)-SH<LCM). Larger alkoxy groups and substituted alkoxy groups with 7(S)-configuration resulted in weaker antibacterial activities than those of LCM, while 7(S)-7-alkylthio-7-deoxylincomycin was more active than LCM against Gram-positive organisms. As a result, a sulfur atom might be preferable to an oxygen atom at the 7-position for enhancing antibacterial activities. 7(R)-7-deoxy-7-imidazol-2-yl-thiolincomycin had the same antibacterial activity as LCM. Here we planned synthesis of lincomycin analogs possessing a heterocyclic ring via sulfur atom focusing on the 7(S)-configuration at the 7-position, in order to generate unreported antibacterial activity against respiratory infection-related Gram-positive bacteria with erm gene.
Results and Discussion
Design of LCM derivatives
The results of X-ray crystallographic analysis4, 5, 6 have already been reported in application of CLDM. According to three-dimensional information around the 7-position of CLDM, we hypothesized that CLDM had enough three-dimensional space around the 7-position, and it might be able to enhance antibacterial activities by filling the space with an appropriate substituent. So we designed 7(S)-LCM derivatives possessing a hydrophobic heterocyclic ring via sulfur atom and practically synthesized 7(S)-7-arylthio-7-deoxylincomycin by Mitsunobu reaction.
Synthesis of 7(S)-7-arylthio-7-deoxylincomycin derivatives
Synthesis of 7(S)-7-arylthio-7-deoxylincomycin derivatives is outlined in Scheme 1. We first prepared a key intermediate 141 derived from LCM in two steps in order to construct the same configuration at the 7-position as CLDM in final target molecules. Our synthesis began with silylation of all OH groups of LCM, and the 7-O-TMS group was selectively deprotected by AcOH to give the key intermediate (1) with an excellent yield. LCM derivatives (2–5, 7–17 and 21–27), which possess a heterocyclic ring at the C-7 position via sulfur atom, were synthesized from 1 with the corresponding thiol under the Mitsunobu condition (Methods A and C).42, 43 Compounds 638 and 18–20 possessing a phenyl thiol group were synthesized by Method B in application of the corresponding disulfide. Furthermore, compound 2838 was prepared by allylation of 5 under the NaOMe condition to investigate structure–activity relationship (SAR) of an allyl group. As a part of chemical modification of the benzothiazole group, 29 was also prepared by reduction of the nitro group of 17 using SnCl2·H2O-NaBH4. Next, 30 was synthesized by SN2 reaction of CLDM with the corresponding thiol under the basic condition to confirm the effect on antibacterial activity by configuration at the C-7 position. On the other hand, the nitrophenyl derivatives (18–20), which were synthesized under the Mitsunobu condition, were reduced to give the corresponding aminophenyl derivatives (31–33). Because compound 33 was the most potent compared with 31 or 32, 33 was converted to compound 34. A Boc group and three trimethylsilyl (TMS) groups of 35 prepared by the Mitsunobu condition were simultaneously deprotected to give a desired compound 36. TMS protection of 21 gave 37, which was reacted with 2-methoxyacetyl chloride with triethylamine, and then deprotected to provide a methoxyacethyl derivative (38).
On the other hand, Mitsunobu conditions still had problems, and thus compounds 18, 20 and 40 were synthesized in only low yields. So compounds 20, 40 and 41 were synthesized by SN2 reactions with the corresponding thiol in good yields under the basic conditions in application of a methanesulfonate 39 synthesized from 1. SAR of novel LCM derivatives are shown in Tables 2, 3, 4, 5.
SAR analysis of LCM derivatives possessing a heterocyclic ring at the C-7 position via sulfur atom
Antibacterial activity of LCM derivatives, which possessed a heterocyclic ring, the benzene ring or an allyl group at the C-7 position via sulfur atom, is shown in Table 2. Compound 2 was shown to be slightly more potent against H. influenzae than CLDM. Compound 3 had weak activity against S. pyogenes with erm gene, although CLDM did not have any activities against the pathogen. So we pursued further optimization at the 7-position. As a result, derivatives possessing a phenyl, thienyl or 1,3,4-thiadiazolyl group did not exhibit remarkable activity against S. pyogenes with erm gene.
Filling a space around the C-7 position might enhance antibacterial activities against resistant S. pyogenes with erm gene. Thus we selected 3 as a fundamental bicycle framework for further optimization. Additionally, 6 and 11 were selected as general examples of an isolated 6-membered ring and an isolated 5-membered ring, respectively. Next we tried to enhance antibacterial activities by introducing a substitute to the selected three frameworks.
SAR analysis of LCM derivatives possessing a benzothiazol-2-ylthio group at the 7-position
LCM derivatives having a benzothiazoyl or a thiazoropyridyl group at the 7-position were synthesized and their antibacterial activities are shown in Table 3. Notably, conversion of 3 to 12, 15 or 16 at the 7-position improved antibacterial activity against S. pneumoniae with erm gene. Especially, antibacterial activity of 16 was significantly enhanced. An amino group might have a role to enhance antibacterial activity and may interact with a certain binding site on 23S rRNA. Compound 29 possessing an amino group at the 5-position in the benzothiazole ring generally exhibited improved antibacterial activity against S. pneumoniae with erm gene compared with 3. On the other hand, we prepared 30 possessing the (R)-configuration at the 7-position to confirm its potency. Based on comparison of antibacterial activities of compound 30 to those of compound 16, we found that (S)-configuration at the 7-position was important for potent antibacterial activities.
SAR analysis of LCM derivatives possessing a phenylthio group at the 7-position
Next we consequently pursued modification of LCM derivatives possessing a substituted phenyl group at the C-7 position via sulfur atom and their antibacterial activities are shown in Table 4. The enhanced antibacterial activities by introducing an amino (16) or a nitro group (15) as shown in Table 3 encouraged us to further explore SAR through introducing an amino or a nitro group at the o-, m- and p-position on the phenyl ring. Unexpectedly, compounds 18–20 and 31–33 showed weaker antibacterial activities against resistant bacteria with erm gene than 15 or 16. Among nitrophenyl and aminophenyl derivatives in Table 4, the para-amino derivative (33) exhibited relatively stronger activities against susceptible S. pneumoniae and H. influenzae. Conversion of the amino group to a methoxycarbonyl group (33 to 40) at the p-position on the phenyl ring improved antibacterial activities against bacteria with erm gene. Based on SAR analysis of compounds 18–20, 31–33, 40 and 41, para-substituted analogs exhibited relatively stronger antibacterial activities against resistant bacteria than o-substituted or m-substituted analogs. Furthermore, 34 possessing a methanesulfonyl group at the amino group of 33 exhibited the most potent antibacterial activity in the phenylthio derivatives, except some strains belonging to S. pneumoniae with erm gene. These results suggest that (i) it is important for a substituent to keep a specific size, length and three-dimensional direction for appropriate binding to rRNA, and (ii) there are several important hydrogen bondings with some functional moieties, such as C=O, N=O, S=O or NH2.
SAR analysis of LCM derivatives possessing a 1,3,4-thiadiazolylthio group at the 7-position
Next we pursued chemical modification of LCM derivatives possessing a substituted 1,3,4-thiadiazole at the C-7 position via sulfur atom and their antibacterial activities are shown in Table 5. We could find several important functional moieties for improvement of activity so far. Then we first introduced an amino group on the 1,3,4-thiadiazole ring. Compound 21 exhibited improved activities against both resistant bacteria with erm gene and H. influenzae compared with 11. We synthesized several compounds 22, 23, 36 and 38, which have an additional group introduced to the amino group of 21. Compound 36 possessing a glycyl moiety as an aliphatic amine had weaker antibacterial activities than 21. Conversion of the glycyl moiety to a methoxyacetyl moiety (36→38) improved antibacterial activities. Furthermore, derivatives 22 and 23 were synthesized and a difluoromethylthiophenyl analog (23) showed stronger activities against S. pneumoniae with erm gene than 21. These results suggest that the phenyl moiety of 23 is an important group and that it enhances antibacterial activities against resistant bacteria with erm gene. We synthesized 27 in order to confirm this hypothesis. As expected, 27 showed relatively stronger antibacterial activities against bacteria with erm gene compared with 21. Finally, we were interested in conversion of a NH-CO bonding to a CO-NH bonding (36 and 38→24–26). Compound 26 also exhibited effective antibacterial activities against bacteria with erm gene. These results suggest that filling a space around the 7-position of LCM has an important role to enhance antibacterial activities by hydrogen bonding, π-π stacking or CH–π interaction to undefined binding site on 23S rRNA.
Conclusion
With a purpose of generating new effective templates against bacteria, we first prepared the key intermediate 141 derived from LCM with two steps to synthesize LCM analogs possessing the same configuration at the 7-position as CLDM in final target molecules. Our LCM derivatives were generally synthesized under Mitsunobu condition with the corresponding thiol from the key intermediate (1).
Compounds 16, 21 and 27 exhibited antibacterial activities against respiratory infection-related Gram-positive bacteria with erm gene, although CLDM did not have any activities against those pathogens. Furthermore, we confirmed that 7(S)-configuration was necessary for enhancing antibacterial activities on the basis of comparison results of configurations of 16 and 30. This work suggests that LCM derivatives may overcome resistant bacteria. SAR analysis, as indicated in this paper, would be useful for further medicinal chemistry in application of LCM derivatives. Our synthetic research in LCM derivatives is in progress.
Experimental Procedure
General methods
1H NMR spectra were measured with a BRUKER Ascend 400 NMR spectrometer (BRUKER corporation, Coventry, UK) for 400 MHz, JEOL JNM-GSX 400 NMR spectrometer (JEOL Ltd.,Tokyo, Japan) for 400 MHz or a Varian Gemini 300 NMR spectrometer (Varian Inc., Palo Alto, CA, USA) for 300 MHz in CDCl3 or CD3OD. TMS (0 p.p.m.) in CDCl3 or CD3OD was used as an internal reference standard. Mass spectra (MS) were obtained on a JEOL JMS-700 mass spectrometer (JEOL Ltd.) or Agilent Technologies 6530-Q-TOF LC/MS mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). The optical rotations were recorded with Jasco P-2300 digital polarimeter (Jasco corporation, Tokyo, Japan). Column chromatography was performed with silica gel (Wakogel C200, Wako Pure Chemical Industries Ltd., Osaka, Japan). Preparative thin layer chromatography was performed with silica gel (Merck, Darmstadt, Germany: TLC plates Silica gel 60 F254). All organic extracts were dried over anhydrous MgSO4, and the solvent was removed with a rotary evaporator under reduced pressure.
2,3,4-Tris-O-(trimethylsilyl)lincomycin (1)
To a solution of lincomycin (50 g, 123 mmol) in pyridine (200 ml) were added trimethylchlorosilane (90 ml, 704 mmol) and hexamethyldisilazane (65 ml, 310 mmol) and stirred at room temperature (RT) for 2 h, and then it was concentrated under reduced pressure. The residue was diluted with water, then extracted with hexane, washed with water and concentrated under reduced pressure. To a solution of the resulting residue in methanol (150 ml) was added 80% aqueous acetic acid (22.5 ml) stirred at RT for 16 h. The mixture was diluted with saturated aqueous NaHCO3 (30 ml) and concentrated under reduced pressure. The residue was diluted with water and hexane, then extracted with hexane, washed with water, dried over MgSO4 and concentrated under reduced pressure. The title compound was obtained as a colorless solid (69.5 g, 91%). ESI-MS (m/z) 623 (M+H)+ as C27H58N2O6SSi3; 1H NMR (400 MHz, chloroform-d) δ 0.14 (s, 18 H), 0.18 (s, 9 H), 0.85–0.93 (m, 3 H), 1.14 (d, J=6.4 Hz, 3 H), 1.22–1.35 (m, 4 H), 1.79–1.90 (m, 1 H), 1.92–2.07 (m, 3 H), 2.09 (s, 3 H), 2.38 (s, 3 H), 3.00 (dd, J=10.9, 3.9 Hz, 1 H), 3.07 (br d, J=1.6 Hz, 1 H), 3.12–3.21 (m, 1 H), 3.59 (dd, J=9.5, 2.5 Hz, 1 H), 3.80 (br d, J=2.5 Hz, 1 H), 4.00 (d, J=9.5 Hz, 1 H), 4.04–4.13 (m, 1 H), 4.15 (dd, J=9.5, 5.6 Hz, 1 H), 4.27–4.33 (m, 1 H), 5.21 (d, J=5.6 Hz, 1 H), 7.42 (d, J=9.8 Hz, 1 H).
7(S)-7-(Benzo[d]oxazol-2-ylthio)-7-deoxylincomycin (2)
To a solution of compound 1 (240 mg, 0.39 mmol) in tetrahydrofuran (THF; 5 ml) at 0 °C were added triphenylphosphine (150 mg, 0.57 mmol), diethylazodicarboxylate (0.10 ml, 0.55 mmol) and benzo[d]oxazole-2-thiol (85 mg, 0.56 ml) and stirred at 0 °C for 1 h. The mixture was stirred at RT for 16 h. The mixture was diluted with 2 N HCl (1 ml)-MeOH (1 ml) and then stirred at RT for 30 min and concentrated under reduced pressure. The resulting residue was dissolved by water and washed with diethyl ether. The mixture was added to NaHCO3 (150 mg), then extracted with ethyl acetate, washed with water, dried over MgSO4 and concentrated under reduced pressure. The resulting residue was purified by preparative TLC (CHCl3/CH3OH/28% aq NH4OH=20/1/0.1) to obtain the title compound as a colorless solid (147.6 mg, 71%). [α]D26 +77.6° (c 0.77, MeOH); ESI-MS (m/z) 540 (M+H)+ as C25H37N3O6S2; TOF-ESI-HRMS (M+H)+ calcd for C25H37N3O6S2: 540.2202, found: 540.2204; 1H NMR (400 MHz, methanol-d4) δ 0.85–0.97 (m, 3 H), 1.26–1.40 (m, 4 H), 1.61 (d, J=6.8 Hz, 3 H), 1.77–1.85 (m, 1 H), 1.87 (s, 3 H), 1.97–2.09 (m, 2 H), 2.12–2.28 (m, 1 H), 2.35 (s, 3 H), 2.98 (dd, J=10.5, 5.2 Hz, 1 H), 3.21 (dd, J=8.5, 6.2 Hz, 1 H), 3.56 (dd, J=10.2, 3.2 Hz, 1 H), 3.83 (br d, J=3.2 Hz, 1 H), 4.10 (dd, J=10.2, 5.6 Hz, 1 H), 4.43 (br d, J=9.8 Hz, 1 H), 4.45 (dq, J=6.8, 3.2 Hz, 1 H), 4.64 (dd, J=9.8, 3.2 Hz, 1 H), 5.24 (d, J=5.6 Hz, 1 H), 7.27–7.36 (m, 2 H), 7.49–7.60 (m, 2 H).
7(S)-7-(Benzo[d]thiazol-2-ylthio)-7-deoxylincomycin (3)
Compound 1 (320 mg, 0.51 mmol) and benzo[d]thiazole-2-thiol (250 mg, 1.49 mmol) were treated according to the similar procedure as described for the preparation of 2 to afford 3 (225.5 mg, 79%) as a colorless solid. [α]D26 74.1° (c 1.05, MeOH); ESI-MS (m/z) 556 (M+H)+ as C25H37N3O5S3; TOF-ESI-HRMS (M+H)+ calcd for C25H37N3O5S3: 556.1974, found: 556.1975; 1H NMR (400 MHz, methanol-d4) δ 0.85–0.98 (m, 3 H), 1.24–1.40 (m, 4 H), 1.59 (d, J=6.8 Hz, 3 H), 1.78–1.90 (m, 1 H), 1.83 (s, 3 H), 1.98–2.10 (m, 2 H), 2.12–2.27 (m, 1 H), 2.34 (s, 3 H), 3.01 (dd, J=10.4, 5.3 Hz, 1 H), 3.19 (dd, J=8.6, 6.1 Hz, 1 H), 3.58 (dd, J=10.3, 3.3 Hz, 1 H), 3.82 (br dd, J=3.3, 0.6 Hz, 1 H), 4.11 (dd, J=10.3, 5.6 Hz, 1 H), 4.43 (br dd, J=9.7, 0.6 Hz, 1 H), 4.52 (dq, J=6.8, 3.1 Hz, 1 H), 4.62 (dd, J=9.7, 3.1 Hz, 1 H), 5.25 (d, J=5.6 Hz, 1 H), 7.35 (ddd, J=8.1, 7.2, 1.2 Hz, 1 H), 7.45 (ddd, J=8.1, 7.2, 1.2 Hz, 1 H), 7.81–7.89 (m, 2 H).
7(S)-7-(1H-Benzo[d]imidazol-2-ylthio)-7-deoxylincomycin (4)
Compound 1 (240 mg, 0.39 mmol) and 1H-benzo[d]imidazole-2-thiol (87.9 mg, 0.59 mmol) were treated according to the similar procedure as described for the preparation of 2 to afford 4 (157.7 mg, 76%) as a colorless solid. [α]D27 +91.9° (c 1.06, MeOH); ESI-MS (m/z) 539 (M+H)+ as C25H38N4O5S2; TOF-ESI-HRMS (M+H)+ calcd for C25H38N4O5S2: 539.2362, found: 539.2361; 1H NMR (400 MHz, methanol-d4) δ 0.87–0.97 (m, 3 H), 1.25–1.38 (m, 4 H), 1.47 (d, J=7.1 Hz, 3 H), 1.72–1.83 (m, 1 H), 1.90–2.05 (m, 2 H), 1.93 (s, 3 H), 2.14–2.28 (m, 1 H), 2.24 (s, 3 H), 3.01 (dd, J=10.3, 5.4 Hz, 1 H), 3.13 (dd, J=8.6, 6.2 Hz, 1 H), 3.60 (dd, J=10.3, 3.4 Hz, 1 H), 3.84 (br d, J=3.4 Hz, 1 H), 4.12 (dd, J=10.3, 5.6 Hz, 1 H), 4.16 (dq, J=7.1, 3.2 Hz, 1 H), 4.43–4.49 (m, 1 H), 4.51 (dd, J=9.5, 3.2 Hz, 1 H), 5.24 (d, J=5.6 Hz, 1 H), 7.19–7.26 (m, 2 H), 7.50 (br s, 2 H).
7(S)-S-(7-Deoxylincomycin-7-yl)benzothioate (5)
To a solution of compound 1 (500 mg, 0.8 mmol) in toluene (5 ml) at 0 °C were added triphenylphosphine (316 mg, 1.20 mmol), diethylazodicarboxylate (0.22 ml, 1.20 mmol) and benzothioic S-acid (172 mg, 1.24 mmol) and stirred at RT for 3 h. The mixture was diluted with 2 N HCl (2 ml) and concentrated under reduced pressure. The resulting residue was dissolved by water and washed with diethyl ether. The mixture was added NaHCO3 (150 mg), then extracted with ethyl acetate, washed with water, dried over MgSO4 and concentrated under reduced pressure. The resulting residue was purified by preparative TLC (CHCl3/CH3OH/28% aq NH4OH=9/1/0.1) to obtain the title compound as a colorless solid (100 mg, 24%). ESI-MS (m/z) 527 (M+H)+ as C25H38N2O6S2; 1H NMR (400 MHz, methanol-d4) δ 0.85–0.98 (m, 3 H), 1.23–1.39 (m, 4 H), 1.44 (d, J=6.8 Hz, 3 H), 1.83–1.92 (m, 1 H), 1.88 (s, 3 H), 1.98–2.07 (m, 1 H), 2.07–2.15 (m,1 H), 2.15–2.28 (m, 1 H), 2.43 (s, 3 H), 3.07 (dd, J=10.5, 5.1 Hz, 1 H), 3.27 (dd, J=8.3, 5.8 Hz, 1 H), 3.55 (dd, J=10.2, 3.3 Hz, 1 H), 3.78–3.85 (m, 1 H), 4.11 (dd, J=10.2, 5.7 Hz, 1 H), 4.23–4.32 (m, 2 H), 4.58 (dd, J=9.7, 3.2 Hz, 1 H), 5.25 (d, J=5.7 Hz, 1 H), 7.42–7.55 (m, 2 H), 7.59–7.68 (m, 1 H), 7.91–8.00 (m, 2 H).
7(S)-7-Deoxy-7-phenylthiolincomycin (6)
To a solution of compound 1 (1.0 g, 1.6 mmol) in THF (15 ml) at 0 °C were added tributylphosphine (971 mg, 4.8 mmol), diethylazodicarboxylate (0.59 ml, 3.2 mmol) and 1,2-diphenyldisulfide (530 mg, 2.4 mmol) and stirred at RT for 24 h. The mixture was diluted with 2 N HCl (1 ml) and stirred at RT for 30 min and then concentrated under reduced pressure. The resulting residue was dissolved by water and washed with diethyl ether. The mixture was added to NaHCO3 (150 mg), then extracted with ethyl acetate, washed with water, dried over MgSO4 and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (CHCl3/CH3OH/28% aq NH4OH=20/1/0.1→9/1/0.1) to obtain the title compound as a colorless solid (724.0 mg, 91%). [α]D26 +111.1° (c 0.63, MeOH); ESI-MS (m/z) 499 (M+H)+ as C24H38N2O5S2; TOF-ESI-HRMS (M+H)+ calcd for C24H38N2O5S2: 499.2300, found: 499.2304; 1H NMR (400 MHz, methanol-d4) δ 0.88–0.97 (m, 3 H), 1.29 (d, J=6.9 Hz, 3 H), 1.31–1.41 (m, 4 H), 1.80–1.90 (m, 1 H), 1.95–2.04 (m, 1 H), 2.00 (s, 3 H), 2.05–2.25 (m, 2 H), 2.39 (s, 3 H), 2.98 (dd, J=10.7, 4.6 Hz, 1 H), 3.24 (dd, J=8.3, 5.9 Hz, 1 H), 3.58 (dd, J=10.2, 3.3 Hz, 1 H), 3.74 (br d, J=3.3 Hz, 1 H), 3.86 (qd, J=6.9, 2.6 Hz, 1 H), 4.10 (dd, J=10.2, 5.6 Hz, 1 H), 4.35 (dd, J=9.7, 0.6 Hz, 1 H), 4.41 (dd, J=9.7, 2.6 Hz, 1 H), 5.26 (d, J=5.6 Hz, 1 H), 7.22–7.28 (m, 1 H), 7.29–7.36 (m, 2 H), 7.40–7.46 (m, 2 H).
7(S)-7-Deoxy-7-(pyridin-4-ylthio)lincomycin (7)
Compound 1 (100 mg, 0.16 mmol) and pyridine-4-thiol (27.6 mg, 0.25 mmol) were treated according to the similar procedure as described for the preparation of 2 to afford 7 (99.8 mg, 40%) as a colorless solid. [α]D26 +88.3° (c 0.94, MeOH); ESI-MS (m/z) 500 (M+H)+ as C23H37N3O5S2; TOF-ESI-HRMS (M+H)+ calcd for C23H37N3O5S2: 500.2253, found: 500.2259; 1H NMR (400 MHz, methanol-d4) δ 0.87–0.98 (m, 3 H), 1.28–1.40 (m, 4 H), 1.46 (d, J=7.0 Hz, 3 H), 1.78 (s, 3 H), 1.79–1.89 (m, 1 H), 1.96–2.11 (m, 2 H), 2.13–2.25 (m, 1 H), 2.39 (s, 3 H), 2.98 (dd, J=10.5, 4.9 Hz, 1 H), 3.24 (dd, J=8.4, 5.9 Hz, 1 H), 3.57 (dd, J=10.2, 3.2 Hz, 1 H), 3.80 (br dd, J=3.2, 0.8 Hz, 1 H), 4.09 (dd, J=10.2, 5.6 Hz, 1 H), 4.11 (dq, J=7.0, 2.9 Hz, 1 H), 4.37 (br dd, J=9.6, 0.8 Hz, 1 H), 4.59 (dd, J=9.6, 2.9 Hz, 1 H), 5.23 (d, J=5.6 Hz, 1 H), 7.33–7.39 (m, 2 H), 8.29–8.35 (m, 2 H).
7(S)-7-Deoxy-7-(pyrimidin-4-ylthio)lincomycin (8)
Compound 1 (100 mg, 0.16 mmol) and pyrimidine-4-thiol (27.9 mg, 0.25 mmol) were treated according to the similar procedure as described for the preparation of 2 to afford 8 (124 mg, 50%) as a colorless solid. [α]D27 +85.0° (c 1.53, MeOH); ESI-MS (m/z) 501 (M+H)+ as C22H36N4O5S2; TOF-ESI-HRMS (M+H)+ calcd for C22H36N4O5S2: 501.2205, found: 501.2208; 1H NMR (400 MHz, methanol-d4) δ 0.87–0.98 (m, 3 H), 1.26–1.41 (m, 4 H), 1.48 (d, J=6.7 Hz, 3 H), 1.79 (s, 3 H), 1.79–1.90 (m, 1 H), 1.97–2.12 (m, 2 H), 2.13–2.26 (m, 1 H), 2.37 (s, 3 H), 3.01 (dd, J=10.5, 5.1 Hz, 1 H), 3.24 (dd, J=8.4, 6.0 Hz, 1 H), 3.55 (dd, J=10.2, 3.2 Hz, 1 H), 3.80 (br dd, J=3.2, 0.7 Hz, 1 H), 4.10 (dd, J=10.2, 5.6 Hz, 1 H), 4.34 (br dd, J=9.5 Hz, 0.7, 1 H), 4.49–4.60(m, 2 H), 5.23 (d, J=5.6 Hz, 1 H), 7.40 (dd, J=5.6, 1.5 Hz, 1 H), 8.36 (br dd, J=5.6, 0.4 Hz, 1 H), 8.87 (br dd, J=1.5, 0.4 Hz, 1 H).
7(S)-7-Deoxyl-7-(thiophen-2-ylthio)lincomycin (9)
Compound 1 (240 mg, 0.39 mmol) and thiophene-2-thiol (100 mg, 0.86 mmol) were treated according to the similar procedure as described for the preparation of 2 to afford 9 (19.4 mg, 10%) as a colorless solid. [α]D26 +140.7° (c 0.47, MeOH); ESI-MS (m/z) 505 (M+H)+ as C22H36N2O5S3; TOF-ESI-HRMS (M+H)+ calcd for C22H36N2O5S3: 505.1865, found: 505.1863; 1H NMR (400 MHz, methanol-d4) δ 0.89–0.98 (m, 3 H), 1.27 (d, J=7.1 Hz, 3H), 1.30–1.41 (m, 4 H), 1.78–1.89 (m, 1 H), 1.92–2.00 (m, 1 H), 2.01–2.08 (m, 1 H), 2.08–2.19 (m, 1 H), 2.21 (s, 3 H), 2.33 (s, 3 H), 2.96 (dd, J=10.7, 4.6 Hz, 1 H), 3.20 (dd, J=8.1, 5.6 Hz, 1 H), 3.54–3.65 (m, 2 H), 3.73 (br d, J=2.8 Hz, 1 H), 4.11 (dd, J=10.3, 5.6 Hz, 1 H), 4.34 (dd, J=9.8, 2.9 Hz, 1 H), 4.37–4.43 (m, 1 H), 5.29 (d, J=5.6 Hz, 1 H), 7.06 (dd, J=5.4, 3.5 Hz, 1H), 7.22 (dd, J=3.5, 1.2 Hz, 1 H), 7.55 (dd, J=5.4, 1.2 Hz, 1 H).
7(S)-7-Deoxy-7-(thiazol-2-ylthio)lincomycin (10)
Compound 1 (240 mg, 0.39 mmol) and thiazole-2-thiol (43.0 mg, 0.37 mmol) were treated according to the similar procedure as described for the preparation of 2 to afford 10 (13.6 mg, 7%) as a colorless solid. [α]D26 +109.5° (c 0.67, MeOH); ESI-MS (m/z) 506 (M+H)+ as C21H35N3O5S3; TOF-ESI-HRMS (M+H)+ calcd for C21H35N3O5S3: 506.1817, found: 506.1802; 1H NMR (400 MHz, methanol-d4) δ 0.88–0.97 (m, 3 H), 1.27–1.40 (m, 4 H), 1.61 (d, J=7.0 Hz, 3 H), 1.79–1.92 (m, 1H), 1.95–2.14 (m, 2H), 2.01 (s, 3H), 2.15–2.29 (m, 1 H), 2.35 (s, 3 H), 3.01 (dd, J=10.4, 5.3 Hz, 1 H), 3.26 (dd, J=8.6, 6.1 Hz, 1 H), 3.57 (dd, J=10.3, 3.3 Hz, 1 H), 3.78 (br d, J=3.3 Hz, 1 H), 4.10 (dd, J=10.3, 5.6 Hz, 1 H), 4.13 (dq, J=7.0, 3.1 Hz, 1 H), 4.39 (br d, J=9.8 Hz, 1 H), 4.51 (dd, J=9.8, 3.1 Hz, 1 H), 5.25 (d, J=5.6 Hz, 1 H), 7.54 (d, J=3.5 Hz, 1 H), 7.73 (d, J=3.5 Hz, 1 H).
7(S)-7-Deoxy-7-(1,3,4-thiadiazol-2-ylthio)lincomycin (11)
Compound 1 (240 mg, 0.39 mmol) and 1,3,4-thiadiazole-2-thiol (80.0 mg, 0.68 mmol) were treated according to the similar procedure as described for the preparation of 2 to afford 11 (121.0 mg, 62%) as a colorless solid. [α]D27 +100.2° (c 2.03, MeOH); ESI-MS (m/z) 507 (M+H)+ as C20H34N4O5S3; TOF-ESI-HRMS (M+H)+ calcd for C20H34N4O5S3: 507.1770, found: 507.1773; 1H NMR (400 MHz, methanol-d4) δ 0.87–0.98 (m, 3 H), 1.26–1.42 (m, 4 H), 1.53 (d, J=6.8 Hz, 3 H), 1.80–1.90 (m, 1 H), 1.93 (s, 3 H), 1.97–2.12 (m, 2 H), 2.15–2.28 (m, 1 H), 2.38 (s, 3 H), 3.03 (dd, J=10.5, 5.1 Hz, 1 H), 3.27 (dd, J=8.4, 6.1 Hz, 1 H), 3.57 (dd, J=10.2, 3.2 Hz, 1 H), 3.81 (br dd, J=3.2, 0.8 Hz, 1 H), 4.11 (dd, J=10.2, 5.6 Hz, 1 H), 4.37–4.46 (m, 2 H), 4.60 (dd, J=9.8, 3.2 Hz, 1 H), 5.26 (d, J=5.6 Hz, 1 H), 9.37 (s, 1H).
7(S)-7-(5-Chlorobenzo[d]thiazol-2-ylthio)-7-deoxylincomycin (12)
Compound 1 (160 mg, 0.26 mmol) and 5-chlorobenzo[d]thiazole-2-thiol (160 mg, 0.79 mmol) were treated according to the similar procedure as described for the preparation of 2 to afford 12 (110.6 mg, 73%) as a colorless solid. [α]D26 +52.8° (c 0.25, MeOH); ESI-MS (m/z) 590 (M+H)+ as C25H36ClN3O5S3; TOF-ESI-HRMS (M+H)+ calcd for C25H36ClN3O5S3: 590.1584, found: 590.1581; 1H NMR (400 MHz, methanol-d4) δ 0.87–0.96 (m, 3 H), 1.27–1.39 (m, 4 H), 1.59 (d, J=7.0 Hz, 3 H), 1.78–1.88 (m, 1 H), 1.86 (s, 3 H), 1.97–2.08 (m, 2 H), 2.13–2.27 (m, 1 H), 2.32 (s, 3 H), 2.98 (dd, J=10.5, 5.2 Hz, 1 H), 3.20 (dd, J=8.5, 6.2 Hz, 1 H), 3.56 (dd, J=10.2, 3.2 Hz, 1 H), 3.82 (br dd, J=3.2, 0.9 Hz, 1 H), 4.10 (dd, J=10.2, 5.6 Hz, 1 H), 4.41 (br dd, J=9.7, 0.9 Hz, 1 H), 4.53 (dq, J=7.0, 3.3 Hz, 1 H), 4.61 (dd, J=9.7, 3.3 Hz, 1 H), 5.24 (d, J=5.6 Hz, 1 H), 7.35 (dd, J=8.6, 2.1 Hz, 1 H), 7.81–7.87 (m, 2 H).
7(S)-7-Deoxyl-7-(thiazolo[5,4-c]pyridin-2-ylthio)lincomycin (13)
Compound 1 (320 mg, 0.51 mmol) and thiazolo[5,4-c]pyridine-2-thiol (250 mg, 1.49 mmol) were treated according to the similar procedure as described for the preparation of 2 to afford 13 (205.9 mg, 72%) as a colorless solid. [α]D26 +67.1° (c 0.50, MeOH); ESI-MS (m/z) 505 (M+H)+ as C24H36N4O5S3; TOF-ESI-HRMS (M+H)+ calcd for C24H36N4O5S3: 557.1926, found: 557.1924; 1H NMR (400 MHz, methanol-d4) δ 0.87–0.98 (m, 3 H), 1.27–1.41 (m, 4 H), 1.62 (d, J=6.7 Hz, 3 H), 1.82 (s, 3 H), 1.80–1.91 (m, 1 H), 1.98–2.13 (m, 2 H), 2.15–2.28 (m, 1 H), 2.38 (s, 3 H), 3.03 (dd, J=10.4, 5.3 Hz, 1 H), 3.24 (dd, J=8.6, 6.2 Hz, 1 H), 3.56 (dd, J=10.3, 3.2 Hz, 1 H), 3.83 (br dd, J=3.2, 0.73 Hz, 1 H), 4.11 (dd, J=10.3, 5.6 Hz, 1 H), 4.43 (br dd, J=9.5, 0.73 Hz, 1 H), 4.63–4.72 (m, 2 H), 5.24 (d, J=5.6 Hz, 1 H), 7.82 (dd, J=5.6, 0.9 Hz, 1 H), 8.51 (d, J=5.6 Hz, 1 H), 9.06 (d, J=0.9 Hz, 1 H).
7(S)-7-(6-Cyanobenzo[d]thiazol-2-ylthio)-7-deoxylincomycin (14)
Compound 1 (160 mg, 0.26 mmol) and 6-cyanobenzo[d]thiazole-2-thiol (55 mg, 0.29 mmol) were treated according to the similar procedure as described for the preparation of 2 to afford 14 (98.4 mg, 66%) as a colorless solid. [α]D26 +73.0° (c 1.10, MeOH); ESI-MS (m/z) 581 (M+H)+ as C26H36N4O5S3; TOF-ESI-HRMS (M+H)+ calcd for C26H36N4O5S3: 581.1926, found: 581.1926; 1H NMR (400 MHz, methanol-d4) δ 0.87–0.97 (m, 3 H), 1.25–1.41 (m, 4 H), 1.61 (d, J=6.6 Hz, 3 H), 1.78–1.89 (m, 1 H), 1.82 (s, 3 H), 1.98–2.09 (m, 2 H), 2.14–2.26 (m, 1 H), 2.35 (s, 3 H), 2.98 (dd, J=10.4, 5.1 Hz, 1 H), 3.21 (dd, J=8.5, 6.2 Hz, 1 H), 3.56 (dd, J=10.2, 3.2 Hz, 1 H), 3.82 (br dd, J=3.2, 0.8 Hz, 1 H), 4.10 (dd, J=10.2, 5.7 Hz, 1 H), 4.42 (br dd, J=9.5, 0.8 Hz, 1 H), 4.60–4.70 (m, 2 H), 5.24 (d, J=5.7 Hz, 1 H), 7.76 (dd, J=8.5, 1.7 Hz, 1 H), 7.94 (br dd, J=8.5, 0.6 Hz, 1 H), 8.32 (br dd, J=1.7, 0.6 Hz, 1 H).
7(S)-7-Deoxy-7-(6-nitrobenzo[d]thiazol-2-ylthio)lincomycin (15)
Compound 1 (240 mg, 0.39 mmol) and 6-nitrobenzo[d]thiazole-2-thiol (180 mg, 0.85 mmol) were treated in toluene (5 ml) according to the similar procedure as described for the preparation of 2 to afford 15 (152.7 mg, 66%) as a colorless solid. [α]D29 +67.6° (c 0.60, MeOH); ESI-MS (m/z) 601 (M+H)+ as C25H36N4O7S3; TOF-ESI-HRMS (M+H)+ calcd for C25H36N4O7S3: 601.1824, found: 601.1827; 1H NMR (400 MHz, methanol-d4) δ 0.87–0.98 (m, 3 H), 1.28–1.40 (m, 4 H), 1.62 (d, J=6.7 Hz, 3 H), 1.77–1.89 (m, 1 H), 1.83 (s, 3 H), 1.98–2.09 (m, 2 H), 2.14–2.27 (m, 1 H), 2.36 (s, 3 H), 2.99 (dd, J=10.5, 5.1 Hz, 1 H), 3.22 (dd, J=8.5, 6.2 Hz, 1 H), 3.56 (dd, J=10.1, 3.2 Hz, 1 H), 3.83 (br dd, J=3.2, 0.8 Hz, 1 H), 4.10 (dd, J=10.1, 5.6 Hz, 1 H), 4.43 (br dd, J=9.5, 0.8 Hz, 1 H), 4.62–4.71 (m, 2 H), 5.24 (d, J=5.6 Hz, 1 H), 7.95 (d, J=9.0, 1 H), 8.32 (dd, J=9.0, 2.3, Hz, 1 H), 8.85 (d, J=2.3 Hz, 1 H).
7(S)-7-(6-Aminobenzo[d]thiazol-2-ylthio)-7-deoxylincomycin (16)
Compound 1 (630 mg, 1.01 mmol) and 6-aminobenzo[d]thiazole-2-thiol (300 mg, 1.65 mmol) were treated according to the similar procedure as described for the preparation of 2 to afford 16 (386.7 mg, 67%) as a colorless solid. [α]D26 +89.0° (c 1.11, MeOH); ESI-MS (m/z) 571 (M+H)+ as C25H38N4O5S3; TOF-ESI-HRMS (M+H)+ calcd for C25H38N4O5S3: 571.2083, found: 571.2075; 1H NMR (400 MHz, methanol-d4) δ 0.86–0.97 (m, 3 H), 1.26–1.38 (m, 4 H), 1.52 (d, J=7.0 Hz, 3 H), 1.75–1.89 (m, 1 H), 1.94 (s, 3 H), 1.95–2.11 (m, 2 H), 2.12–2.25 (m, 1 H), 2.32 (s, 3 H), 3.03 (dd, J=10.4, 5.3 Hz, 1 H), 3.17 (dd, J=8.6, 6.2 Hz, 1 H), 3.58 (dd, J=10.2, 3.2 Hz, 1 H), 3.81 (br dd, J=3.2, 0.7 Hz, 1 H), 4.10 (dd, J=10.2, 5.5 Hz, 1 H), 4.27 (dq, J=7.0, 3.1 Hz, 1 H), 4.43 (br dd, J=9.8, 0.7 Hz, 1 H), 4.55 (dd, J=9.8, 3.1 Hz, 1 H), 5.25 (d, J=5.5 Hz, 1 H), 6.85 (dd, J=8.7, 2.3 Hz, 1 H), 7.08 (dd, J=2.3, 0.25 Hz, 1 H), 7.59 (dd, J=8.7, 0.25 Hz, 1 H).
7(S)-7-Deoxy-7-(5-nitrobenzo[d]thiazol-2-ylthio)lincomycin (17)
Compound 1 (320 mg, 0.51 mmol) and 5-nitrobenzo[d]thiazole-2-thiol (120 mg, 0.57 mmol) were treated according to the similar procedure as described for the preparation of 2 to afford 17 (138.8 mg, 45%) as a colorless solid. [α]D26 +58.2° (c 0.81, MeOH); ESI-MS (m/z) 601 (M+H)+ as C25H36N4O7S3; TOF-ESI-HRMS (M+H)+ calcd for C25H36N4O7S3: 601.1824, found: 601.1826; 1H NMR (400 MHz, methanol-d4) δ 0.88–0.97 (m, 3 H), 1.30–1.40 (m, 4 H), 1.62 (d, J=6.8 Hz, 3 H), 1.80–1.90 (m, 1 H), 1.85 (s, 3 H), 1.99–2.10 (m, 2 H), 2.16–2.30 (m, 1 H), 2.36 (s, 3 H), 3.01 (dd, J=10.3, 5.3 Hz, 1 H), 3.25 (dd, J=8.7, 6.2 Hz, 1 H), 3.57 (dd, J=10.3, 3.2 Hz, 1 H), 3.84 (br dd, J=3.2, 0.8 Hz, 1 H), 4.11 (dd, J=10.3, 5.7 Hz, 1 H), 4.42 (br dd, J=9.6, 0.8 Hz, 1 H), 4.61 (dd, J=6.8, 3.3 Hz, 1 H), 4.65 (dd, J=9.6, 3.3 Hz, 1 H), 5.25 (d, J=5.7 Hz, 1 H), 8.08 (d, J=8.8, 1 H), 8.21 (dd, J=8.8, 2.2, Hz, 1 H), 8.64 (br dd, J=2.2, 0.24 Hz, 1 H).
7(S)-7-Deoxy-7-(2-nitrophenylthio)lincomycin (18)
Compound 1 (200 mg, 0.32 mmol) and 1,2-bis(2-nitrophenyl)disulfide (148.5 mg, 0.48 mmol) were treated according to the similar procedure as described for the preparation of 6 to afford 18 (40.1 mg, 23%) as a colorless solid. [α]D27 +69.9° (c 0.91, MeOH); ESI-MS (m/z) 544 (M+H)+ as C24H37N3O7S2; TOF-ESI-HRMS (M+H)+ calcd for C24H37N3O7S2: 544.2151, found: 544.2157; 1H NMR (400 MHz, methanol-d4) δ 0.86–0.99 (m, 3 H), 1.29–1.38 (m, 4 H), 1.38 (d, J=6.8 Hz, 3 H), 1.79 (s, 3 H), 1.81–1.92 (m, 1 H), 1.98–2.14 (m, 2 H), 2.14–2.26 (m, 1 H), 2.42 (s, 3 H), 3.01 (dd, J=10.6, 4.8 Hz, 1 H), 3.28 (dd, J=8.7, 6.1 Hz, 1 H), 3.57 (dd, J=10.3, 3.2 Hz, 1 H), 3.79 (br dd, J=3.2, 0.8 Hz, 1 H), 4.06 (dq, J=6.8, 2.7 Hz, 1 H), 4.08 (dd, J=10.3, 5.6 Hz, 1 H), 4.37 (br dd, J=9.8, 0.8 Hz, 1 H), 4.57 (dd, J=9.8, 2.7 Hz, 1 H), 5.21 (d, J=5.6 Hz, 1 H), 7.38 (ddd, J=8.3, 7.2, 1.2 Hz, 1 H), 7.64 (ddd, J=8.2, 7.2, 1.4 Hz, 1 H), 7.70 (br dd, J=8.2, 1.2 Hz, 1 H), 8.05 (dd, J=8.3, 1.4 Hz, 1 H).
7(S)-7-Deoxy-7-(3-nitrophenylthio)lincomycin (19)
Compound 1 (1.0 g, 1.6 mmol) and 1,2-bis(3-nitrophenyl)disulfide (742 mg, 2.4 mmol) were treated according to the similar procedure as described for the preparation of 6 to afford 19 (366.5 mg, 42%) as a colorless solid. [α]D29 +75.2° (c 0.48, MeOH); ESI-MS (m/z) 544 (M+H)+ as C24H37N3O7S2; TOF-ESI-HRMS (M+H)+ calcd for C24H37N3O7S2: 544.2151, found: 544.2148; 1H NMR (400 MHz, methanol-d4) δ 0.89–0.97 (m, 3 H), 1.30–1.37 (m, 4 H), 1.38 (d, J=6.8 Hz, 3 H), 1.80–1.91 (m, 1 H), 1.93 (s, 3 H), 1.97–2.12 (m, 2 H), 2.13–2.24 (m, 1 H), 2.40 (s, 3 H), 2.99 (dd, J=10.6, 4.8 Hz, 1 H), 3.24 (dd, J=8.3, 5.9 Hz, 1 H), 3.58 (dd, J=10.2, 3.3 Hz, 1 H), 3.79 (br dd, J=3.3, 0.8 Hz, 1 H), 3.99 (dq, J=6.8, 2.8 Hz, 1 H), 4.10 (dd, J=10.2, 5.6 Hz, 1 H), 4.36 (br dd, J=9.5, 0.8 Hz, 1 H), 4.52 (dd, J=9.5, 2.8 Hz, 1 H), 5.25 (d, J=5.6 Hz, 1 H), 7.55–7.61 (m, 1 H), 7.81 (ddd, J=7.9, 1.8, 0.9 Hz, 1 H), 8.09 (br ddd, J=8.2, 2.2, 0.9 Hz, 1 H), 8.21–8.23 (m, 1 H).
7(S)-7-Deoxy-7-(4-nitrophenylthio)lincomycin (20)
Compound 1 (200 mg, 0.32 mmol) and 1,2-bis(4-nitrophenyl)disulfide (148.5 mg, 0.48 mmol) were treated according to the similar procedure as described for the preparation of 6 to afford 20 (29.7 mg, 17%) as a colorless solid. [α]D26 +67.1° (c 0.29, MeOH); ESI-MS (m/z) 544 (M+H)+ as C24H37N3O7S2; TOF-ESI-HRMS (M+H)+ calcd for C24H37N3O7S2: 544.2151, found: 544.2151; 1H NMR (400 MHz, methanol-d4) δ 0.87–0.98 (m, 3 H), 1.30–1.39 (m, 4 H), 1.44 (d, J=6.8 Hz, 3 H), 1.79–1.90 (m, 1 H), 1.81 (s, 3 H), 1.97–2.12 (m, 2 H), 2.13–2.26 (m, 1 H), 2.40 (s, 3 H), 3.00 (dd, J=10.6, 5.0 Hz, 1 H), 3.25 (dd, J=8.4, 6.1 Hz, 1 H), 3.57 (dd, J=10.2, 3.2 Hz, 1 H), 3.80 (br dd, J=3.2, 0.8 Hz, 1 H), 4.09 (dd, J=10.2, 5.6 Hz, 1 H), 4.03–4.16 (m, 1 H), 4.37 (br dd, J=9.7, 0.8 Hz, 1 H), 4.58 (dd, J=9.7, 2.9 Hz, 1 H), 5.24 (d, J=5.6 Hz, 1 H), 7.51–7.57 (m, 2 H), 8.13–8.19 (m, 2 H).
7(S)-7-(5-Amino-1,3,4-thiadiazol-2-ylthio)-7-deoxylincomycin (21)
To a solution of compound 1 (240 mg, 0.39 mmol) in THF (5 ml) at 0 °C were added triphenylphosphine (150 mg, 0.57 mmol), diethylazodicarboxylate (0.1 ml, 0.55 mmol) and t-butyl (5-mercapto-1,3,4-thiadiazol-2-yl)carbamate (130 mg, 0.56 mmol) and stirred at RT for 17 h. The mixture was concentrated under reduced pressure and diluted with saturated aqueous NaHCO3 (10 ml), extracted with ethyl acetate, washed with water, dried over MgSO4 and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (hexane/ethyl acetate) to obtain 7(S)-7-(5-amino-1,3,4-thiadiazol-2-ylthio)-7-deoxy-2,3,4-tris-O-(trimethylsilyl)lincomycin as a colorless solid (271.3 mg, 84%). 7(S)-7-(5-Amino-1,3,4-thiadiazol-2-ylthio)-7-deoxy-2,3,4-tris-O-(trimethylsilyl)lincomycin (271.3 mg, 0.32 mmol) in 90% aqueous trifluoroacetic acid (5 ml) was kept at RT for 1 h. The mixture was concentrated under reduced pressure and diluted with saturated aqueous NaHCO3 (10 ml) and then extracted with ethyl acetate, washed with water, dried over MgSO4 and concentrated under reduced pressure. The resulting residue was purified by preparative TLC (CHCl3/CH3OH/28% aq NH4OH=5/1/0.1) to obtain the title compound as a colorless solid (111.4 mg, 66%). [α]D26 +139.8° (c 0.28, MeOH); ESI-MS (m/z) 520 (M+H)+ as C20H35N5O5S3; TOF-ESI-HRMS (M+H)+ calcd for C20H35N5O5S3: 522.1879, found: 522.1877; 1H NMR (400 MHz, methanol-d4) δ 0.87–0.97 (m, 3 H), 1.27–1.38 (m, 4 H), 1.41 (d, J=7.0 Hz, 3 H), 1.76–1.90 (m, 1 H), 1.92–2.07 (m, 2 H), 2.11 (s, 3 H), 2.14–2.26 (m, 1 H), 2.33 (s, 3 H), 2.97 (dd, J=10.5, 4.9 Hz, 1 H), 3.26 (dd, J=8.6, 6.2 Hz, 1 H), 3.57 (dd, J=10.3, 3.2 Hz, 1 H), 3.74–3.78 (m, 1 H), 3.94 (dq, J=7.0, 2.9 Hz, 1 H), 4.10 (dd, J=10.3, 5.6 Hz, 1 H), 4.39 (br dd, J=9.8, 0.5 Hz, 1 H), 4.45 (dd, J=9.8, 2.9 Hz, 1 H), 5.26 (d, J=5.6 Hz, 1 H). For the qualified analytical purpose, the above colorless solid was further purified by reverse-phase column chromatography (YMC triart C18, 20 × 250 mm, RT, 18.9 ml min−1, 50 mM AcONH4/CH3CN=70/30) and precipitated (MeOH/ethyl acetate) to obtain the highly purified title compound as a colorless solid.
7(S)-7-Deoxy-7-(5-methylamino-1,3,4-thiadiazol-2-ylthio)lincomycin (22)
Compound 1 (240 mg, 0.39 mmol) and t-butyl (5-mercapto-1,3,4-thiadiazol-2-yl)(methyl)carbamate (100 mg, 0.40 mmol) were treated according to the similar procedure as described for the preparation of 21 to afford 22 (147.1 mg, 71% (two steps)) as a colorless solid. [α]D26 +118.4° (c 0.27, MeOH); ESI-MS (m/z) 534 (M+H)+ as C21H37N5O5S3; TOF-ESI-HRMS (M+H)+ calcd for C21H37N5O5S3: 536.2035, found: 536.2039; 1H NMR (400 MHz, methanol-d4) δ 0.87–0.98 (m, 3 H), 1.27–1.38 (m, 4 H), 1.41 (d, J=7.0 Hz, 3 H), 1.77–1.88 (m, 1 H), 1.93–2.01 (m, 1 H), 2.04 (dd, J=10.1, 8.7 Hz, 1 H), 2.12 (s, 3 H), 2.14–2.26 (m, 1 H), 2.33 (s, 3H), 2.93–3.01 (m, 1 H), 2.97 (s, 3 H), 3.25 (dd, J=8.4, 6.2 Hz, 1 H), 3.57 (dd, J=10.3, 3.2 Hz, 1 H), 3.78 (br dd, J=3.2, 0.7 Hz, 1 H), 3.94 (dq, J=7.0, 2.9 Hz, 1 H), 4.10 (dd, J=10.3, 5.6 Hz, 1 H), 4.38 (br dd, J=9.8, 0.7 Hz, 1 H), 4.46 (dd, J=9.8, 2.9 Hz, 1 H), 5.26 (d, J=5.6 Hz, 1 H).
7(S)-7-Deoxy-7-(5-(4-(difluoromethylthio)phenylamino)-1,3,4-thiadiazol-2-ylthio)lincomycin (23)
Compound 1 (240 mg, 0.39 mmol) and 5-(4-(difluoromethylthio)phenylamino)-1,3,4-thiadiazole-2-thiol (120 mg, 0.41 mmol) were treated according to the similar procedure as described for the preparation of 2 to afford 23 (99.5 mg, 38%) as a colorless solid. [α]D24 +96.7° (c 0.78, MeOH); ESI-MS (m/z) 680 (M+H)+ as C27H39F2N5O5S4; TOF-ESI-HRMS (M+H)+ calcd for C27H39F2N5O5S4: 680.1880, found: 680.1876; 1H NMR (400 MHz, methanol-d4) δ 0.85–0.95 (m, 3 H), 1.26–1.39 (m, 4 H), 1.46 (d, J=7.0 Hz, 3 H), 1.78–1.88 (m, 1 H), 1.93–2.06 (m, 2 H), 2.12 (s, 3 H), 2.14–2.28 (m, 1 H), 2.33 (s, 3 H), 2.95–3.02 (m, 1 H), 3.26 (dd, J=8.4, 6.2 Hz, 1 H), 3.59 (dd, J=10.3, 3.3 Hz, 1 H), 3.79 (br d, J=3.3 Hz, 1 H), 4.02–4.10 (m, 1 H), 4.12 (dd, J=10.3, 5.6 Hz, 1 H), 4.41 (br d, J=9.8 Hz, 1 H), 4.49 (dd, J=9.8, 3.6 Hz, 1 H), 5.28 (d, J=5.6 Hz, 1 H), 7.00 (t, J=56.8 Hz, 1 H), 7.52–7.58 (m, 2 H), 7.64–7.70 (m, 2 H).
7(S)-7-(5-Carbamoyl-1,3,4-thiadiazol-2-ylthio)-7-deoxylincomycin (24)
Compound 1 (240 mg, 0.39 mmol), and 5-mercapto-1,3,4-thiadiazole-2-carboxamide (95 mg, 0.59 mmol) were treated at 50 °C according to the similar procedure as described for the preparation of 2 to afford 24 (67.8 mg, 32%) as a colorless solid. [α]D26 +83.6° (c 0.69, MeOH); ESI-MS (m/z) 550 (M+H)+ as C21H35N5O6S3; TOF-ESI-HRMS (M+H)+ calcd for C21H35N5O6S3: 550.1828, found: 550.1829; 1H NMR (400 MHz, methanol-d4) δ 0.86–0.97 (m, 3 H), 1.30–1.41 (m, 4 H), 1.57 (d, J=7.0 Hz, 3 H), 1.78–1.89 (m, 1 H), 1.94 (s, 3 H), 1.97–2.09 (m, 2 H), 2.13–2.26 (m, 1 H), 2.37 (s, 3 H), 2.99 (dd, J=10.5, 5.1 Hz, 1 H), 3.24 (dd, J=8.5, 6.2 Hz, 1 H), 3.56 (dd, J=10.3, 3.2 Hz, 1 H), 3.81 (br dd, J=3.2, 0.8 Hz, 1 H), 4.10 (dd, J=10.3, 5.6 Hz, 1 H), 4.40 (br dd, J=9.7, 0.8 Hz, 1 H), 4.49 (dq, J=7.0, 3.1 Hz, 1 H), 4.61 (dd, J=9.7, 3.1 Hz, 1 H), 5.25 (d, J=5.6 Hz, 1 H). For the qualified analytical purpose, the above colorless solid was further purified by reverse-phase column chromatography (YMC triart C18, 20 × 250 mm, RT, 18.9 ml min−1, 50 mM AcONH4/CH3CN=50/50) to obtain the highly purified title compound as a colorless solid.
7(S)-7-Deoxy-7-(5-methylcarbamoyl-1,3,4-thiadiazol-2-ylthio)lincomycin (25)
Compound 1 (240 mg, 0.39 mmol) and 5-mercapto-N-methyl-1,3,4-thiadiazole-2-carboxamide (100 mg, 0.57 mmol) were treated according to the similar procedure as described for the preparation of 2 to afford 25 (26.1 mg, 12%) as a colorless solid. [α]D26 +81.8° (c 1.29, MeOH); ESI-MS (m/z) 564 (M+H)+ as C22H37N5O6S3; TOF-ESI-HRMS (M+H)+ calcd for C22H37N5O6S3: 564.1984, found: 564.1977; 1H NMR (400 MHz, methanol-d4) δ 0.85–0.98 (m, 3 H), 1.26–1.42 (m, 4 H), 1.56 (d, J=6.8 Hz, 3 H), 1.78–1.89 (m, 1 H), 1.94 (s, 3 H), 1.97–2.12 (m, 2 H), 2.13–2.27 (m, 1 H), 2.38 (s, 3 H), 2.94 (s, 3 H), 3.00 (dd, J=10.5, 5.1 Hz, 1 H), 3.25 (dd, J=8.6, 6.1 Hz, 1 H), 3.56 (dd, J=10.2, 3.2 Hz, 1 H), 3.79–3.84 (m, 1 H), 4.10 (dd, J=10.2, 5.6 Hz, 1 H), 4.40 (br d, J=9.8 Hz, 1 H), 4.48 (dq, J=6.8, 3.1 Hz, 1 H), 4.62 (dd, J=9.8, 3.1 Hz, 1 H), 5.25 (d, J=5.6 Hz, 1 H). For the qualified analytical purpose, the above colorless solid was further purified by reverse-phase column chromatography (YMC triart C18, 20 × 250 mm, RT, 11.3 ml min−1, 0.1% aq. TFA/CH3CN=60/40) to obtain the highly purified title compound as a colorless solid.
7(S)-7-Deoxy-7-(5-(2-(pyrrolidin-1-yl)ethylcarbamoyl)-1,3,4-thiadiazol-2-ylthio)lincomycin (26)
Compound 1 (240 mg, 0.39 mmol) and 5-(2-(pyrrolidin-1-yl)ethylcarbamoyl)-1,3,4-thiadiazole-2-thiol (130 mg, 0.50 mmol) were treated according to the similar procedure as described for the preparation of 2 to afford 26 (69.8 mg, 28%) as a colorless solid. [α]D27 +78.0° (c 0.54, MeOH); ESI-MS (m/z) 647 (M+H)+ as C27H46N6O6S3; TOF-ESI-HRMS (M+H)+ calcd for C27H46N6O6S3: 647.2719, found: 647.2716; 1H NMR (400 MHz, methanol-d4) δ 0.87–0.97 (m, 3 H), 1.28–1.39 (m, 4 H), 1.56 (d, J=7.0 Hz, 3 H), 1.77–1.90 (m, 5 H), 1.94 (s, 3 H), 1.97–2.12 (m, 2 H), 2.13–2.26 (m, 1 H), 2.37 (s, 3 H), 2.57–2.67 (m, 4 H), 2.73 (t, J=6.7 Hz, 2 H), 2.98 (dd, J=10.5, 5.1 Hz, 1 H), 3.24 (dd, J=8.5, 6.2 Hz, 1 H), 3.51–3.60 (m, 3 H), 3.81 (br dd, J=3.2, 0.8 Hz, 1 H), 4.10 (dd, J=10.2, 5.6 Hz, 1 H), 4.40 (br dd, J=9.7, 0.8 Hz, 1 H), 4.48 (dq, J=7.0, 3.1 Hz, 1 H), 4.62 (dd, J=9.7, 3.1 Hz, 1 H), 5.25 (d, J=5.6 Hz, 1 H).
7(S)-7-Deoxy-7-(5-phenyl-1,3,4-thiadiazol-2-ylthio)lincomycin (27)
Compound 1 (240 mg, 0.39 mmol) and 5-phenyl-1,3,4-thiadiazole-2-thiol (100 mg, 0.51 mmol) in toluene (5 ml) were treated according to the similar procedure as described for the preparation of 2 to afford 27 (46.5 mg, 21%) as a colorless solid. [α]D27 +157.2° (c 1.47, CHCl3); ESI-MS (m/z) 583 (M+H)+ as C26H38N4O5S3; TOF-ESI-HRMS (M+H)+ calcd for C26H38N4O5S3: 583.2083, found: 583.2086; 1H NMR (400 MHz, methanol-d4) δ 0.86–0.97 (m, 3 H), 1.26–1.38 (m, 4 H), 1.57 (d, J=7.0 Hz, 3 H), 1.79–1.91 (m, 1 H), 1.96–2.11 (m, 2 H), 2.03 (s, 3 H), 2.15–2.28 (m, 1 H), 2.38 (s, 3 H), 3.03 (dd, J=10.5, 5.1 Hz, 1 H), 3.27 (dd, J=8.5, 6.1 Hz, 1 H), 3.58 (dd, J=10.3, 3.2 Hz, 1 H), 3.83 (br dd, J=3.2, 0.7 Hz, 1 H), 4.12 (dd, J=10.3, 5.6 Hz, 1 H), 4.36–4.46 (m, 2 H), 4.60 (dd, J=9.7, 3.2 Hz, 1 H), 5.27 (d, J=5.6 Hz, 1 H), 7.49–7.58 (m, 3 H), 7.89–7.95 (m, 2 H).
7(S)-7-Allylthio-7-deoxylincomycin (28)
To a solution of compound 5 (83.2 mg, 0.16 mmol) in methanol (1 ml) were added allyl iodide (26.5 mg, 0.16 mmol) and 28% sodium methoxide in mehtanol (0.56 ml) and stirred at RT for 14 h. The mixture was diluted with 1 N HCl (1 ml) and concentrated under reduced pressure. The resulting residue was dissolved by water and washed with diethyl ether. The mixture was added to NaHCO3 (150 mg) and then extracted with ethyl acetate, washed with water, dried over MgSO4 and concentrated under reduced pressure. The resulting residue was purified by preparative TLC (CHCl3/CH3OH/28% aq NH4OH=20/1/0.1) to obtain the title compound as a colorless solid (22.0 mg, 30%). [α]D25 +115.3° (c 0.34, MeOH); ESI-MS (m/z) 463 (M+H)+ as C21H38N2O5S2; TOF-ESI-HRMS (M+H)+ calcd for C21H38N2O5S2: 463.2300, found: 463.2295; 1H NMR (400 MHz, methanol-d4) δ 0.89–0.96 (m, 3 H), 1.27–1.40 (m, 4 H), 1.31 (d, J=7.0 Hz, 3 H), 1.82–1.92 (m, 1 H), 2.00 (ddd, J=12.5, 7.6, 4.7 Hz, 1 H), 2.08–2.27 (m, 2 H), 2.19 (s, 3 H), 2.44 (s, 3 H), 3.05 (dd, J=10.5, 4.4 Hz, 1 H), 3.23–3.39 (m, 4 H), 3.56 (dd, J=10.2, 3.3 Hz, 1 H), 3.67–3.74 (m, 1 H), 4.09 (dd, J=10.2, 5.6 Hz, 1 H), 4.21 (br d, J=9.8 Hz,1 H), 4.27 (dd, J=9.8, 2.4 Hz, 1 H), 5.04–5.11 (m, 1 H), 5.19 (dq, J=17.0, 1.4 Hz, 1 H), 5.25 (d, J=5.6 Hz, 1 H), 5.85 (ddt, J=17.0, 10.7, 1.0 Hz, 1 H).
7(S)-7-(5-Aminobenzo[d]thiazol-2-ylthio)-7-deoxylincomycin (29)
To a solution of compound 17 (75.0 mg, 0.12 mmol) in ethanol (3.0 ml) were added SnCl2·H2O (140 mg, 0.62 mmol) and NaBH4 (6.5 mg, 0.17 mmol) and stirred at RT for 3 h. The mixture was concentrated under reduced pressure. The resulting residue was dissolved by ethyl acetate, washed with water, dried over MgSO4 and concentrated under reduced pressure. The resulting residue was purified by preparative TLC (CHCl3/CH3OH/28% aq NH4OH=20/1/0.1) to obtain the title compound as a colorless solid (34.2 mg, 48%). [α]D25 +59.4° (c 0.58, MeOH); ESI-MS (m/z) 571 (M+H)+ as C25H38N4O5S3; TOF-ESI-HRMS (M+H)+ calcd for C25H38N4O5S3: 571.2083, found: 571.2090; 1H NMR (400 MHz, methanol-d4) δ 0.87–0.96 (m, 3 H), 1.25–1.39 (m, 4 H), 1.55 (d, J=7.0 Hz, 3 H), 1.76–1.86 (m, 1 H), 1.88 (s, 3 H), 1.96–2.08 (m, 2 H), 2.12–2.24 (m, 1 H), 2.32 (s, 3 H), 2.99 (dd, J=10.5, 5.2 Hz, 1 H), 3.18 (dd, J=8.5, 6.2 Hz, 1 H), 3.57 (dd, J=10.1, 3.2 Hz, 1H), 3.81 (br dd, J=3.2, 0.7 Hz, 1 H), 4.10 (dd, J=10.1, 5.6 Hz, 1 H), 4.39 (dq, J=7.0, 3.1 Hz, 1 H), 4.42 (br dd, J=9.7, 0.7 Hz, 1 H), 4.58 (dd, J=9.7, 3.1 Hz, 1 H), 5.24 (d, J=5.6 Hz, 1 H), 6.81 (dd, J=8.6, 2.2 Hz, 1H), 7.16–7.18 (m, 1 H), 7.52 (dd, J=8.6, 0.2 Hz, 1 H).
7(R)-7-(6-Aminobenzo[d]thiazol-2-ylthio)-7-deoxylincomycin (30)
To a solution of clindamycin hydrochloride (460 mg, 1.0 mmol) in DMF (5 ml) were added K2CO3 (152 mg, 1.1 mmol) and 6-aminobenzo[d]thiazole-2-thiol (200 mg, 1.1 mmol) and stirred at 100 °C for 16 h. Then it was concentrated under reduced pressure. The residue was diluted with water and then extracted with ethyl acetate, washed with water, dried over MgSO4 and concentrated under reduced pressure. The resulting residue was purified by preparative TLC (CHCl3/CH3OH/28% aq NH4OH=20/1/0.1) to obtain the title compound as a colorless solid (233.3 mg, 41%). [α]D27 +129.5° (c 0.45, MeOH); ESI-MS (m/z) 571 (M+H)+ as C25H38N4O5S3; TOF-ESI-HRMS (M+H)+ calcd for C25H38N4O5S3: 571.2083, found: 571.2079; 1H NMR (400 MHz, methanol-d4) δ 0.87–0.96 (m, 3 H), 1.27–1.39 (m, 4 H), 1.50 (d, J=7.1 Hz, 3 H), 1.75–1.87 (m, 1 H), 1.95–2.01 (m, 1 H), 2.05 (dd, J=10.2, 8.9 Hz, 1 H), 2.17 (s, 3 H), 2.13–2.26 (m, 1 H), 2.39 (s, 3 H), 2.98 (dd, J=10.4, 5.0 Hz, 1 H), 3.21 (dd, J=8.5, 6.2 Hz, 1 H), 3.54 (dd, J=10.2, 3.3 Hz, 1 H), 3.80–3.84 (m, 1 H), 4.11 (dd, J=10.2, 5.6 Hz, 1 H), 4.28 (br dd, J=9.2, 0.6 Hz, 1 H), 4.30 (dq, J=7.1, 3.9 Hz, 1 H), 4.65 (dd, J=9.2, 3.9 Hz, 1 H), 5.29 (d, J=5.6 Hz, 1 H), 6.84 (dd, J=8.7, 2.3 Hz, 1 H), 7.08 (dd, J=2.3, 0.4 Hz, 1 H), 7.57 (dd, J=8.7, 0.4 Hz, 1 H). For the qualified analytical purpose, the above colorless solid was further purified by reverse-phase column chromatography (YMC triart C18, 20 × 250 mm, RT, 18.9 ml min−1, 50 mM AcONH4/CH3CN=35/65) to obtain the highly purified title compound as a colorless solid.
7(S)-7-(2-Aminophenylthio)-7-deoxylincomycin (31)
To a solution of compound 18 (39.6 mg, 0.07 mmol) in ethanol (2 ml) were added SnCl2·H2O (82.1 mg, 0.36 mmol) and NaBH4 (13.7 mg, 5.0 mmol) and stirred at RT for 3 h. To the mixture was added aq NaHCO3 and then extracted with ethyl acetate, washed with water, dried over MgSO4 and concentrated under reduced pressure. The resulting residue was purified by preparative TLC (CHCl3/CH3OH/28% aq NH4OH=9/1/0.1) to obtain the title compound as a colorless solid (10.9 mg, 29%). [α]D28 +74.5° (c 0.33, MeOH); ESI-MS (m/z) 514 (M+H)+ as C24H39N3O5S2; TOF-ESI-HRMS (M+H)+ calcd for C24H39N3O5S2: 514.2409, found: 514.2412; 1H NMR (400 MHz, methanol-d4) δ 0.89–0.97 (m, 3 H), 1.17 (d, J=7.1 Hz, 3 H), 1.28–1.40 (m, 4 H), 1.75–1.85 (m, 1 H), 1.89–1.98 (m, 1 H), 2.04–2.12 (m, 1 H), 2.14–2.23 (m, 1 H), 2.19 (s, 3 H), 2.32 (s, 3 H), 3.01 (dd, J=10.6, 5.0 Hz, 1 H), 3.25 (dd, J=8.3, 5.9 Hz, 1 H), 3.58 (dd, J=10.2, 3.2 Hz, 1 H), 3.67 (dq, J=7.1, 2.8 Hz, 1 H), 3.74 (br dd, J=3.2, 0.5 Hz, 1 H), 4.11 (dd, J=10.2, 5.6 Hz, 1 H), 4.31 (dd, J=9.8, 2.8 Hz, 1 H), 4.38 (br dd, J=9.8, 0.5 Hz, 1 H), 5.27 (d, J=5.6 Hz, 1 H), 6.61–6.67 (m, 1 H), 6.81 (dd, J=8.1, 1.1 Hz, 1 H), 7.13 (ddd, J=8.8, 7.2, 1.5 Hz, 1 H), 7.34 (dd, J=7.7, 1.5 Hz, 1 H).
7(S)-7-(3-Aminophenylthio)-7-deoxylincomycin (32)
Compound 19 (238.2 mg, 0.44 mmol), SnCl2·H2O (494.4 mg, 2.19 mmol) and NaBH4 (82.9 mg, 2.19 mmol) were treated according to the similar procedure as described for the preparation of 31 to afford 32 (36.0 mg, 16%) as a colorless solid. [α]D29 +91.0° (c 0.60, MeOH); ESI-MS (m/z) 514 (M+H)+ as C24H39N3O5S2; TOF-ESI-HRMS (M+H)+ calcd for C24H39N3O5S2: 514.2409, found: 514.2408; 1H NMR (400 MHz, methanol-d4) δ 0.89–0.98 (m, 3 H), 1.31 (d, J=7.0 Hz, 3 H), 1.30–1.40 (m, 4 H), 1.79–1.92 (m, 1 H), 1.93–2.10 (m, 2 H), 2.02 (s, 3 H), 2.11–2.23 (m, 1 H), 2.38 (s, 3 H), 2.97 (dd, J=10.7, 4.6 Hz, 1 H), 3.25 (dd, J=8.1, 5.6 Hz, 1 H), 3.59 (dd, J=10.2, 3.3 Hz, 1 H), 3.72 (br d, J=3.3 Hz, 1 H), 3.75–3.83 (m, 1 H), 4.10 (dd, J=10.2, 5.5 Hz, 1 H), 4.33–4.40 (m, 2 H), 5.26 (d, J=5.5 Hz, 1 H), 6.59 (ddd, J=8.0, 2.2, 0.9 Hz, 1 H), 6.71 (ddd, J=7.7, 1.7, 0.9 Hz, 1 H), 6.78–6.82 (m, 1 H), 6.99–7.06 (m, 1 H).
7(S)-7-(4-Aminophenylthio)-7-deoxylincomycin (33)
Compound 20 (29.3 mg, 0.054 mmol), SnCl2·H2O (60.8 mg, 0.27 mmol) and NaBH4 (10.0 mg, 0.26 mmol) were treated according to the similar procedure as described for the preparation of 31 to afford 33 (9.7 mg, 35%) as a colorless solid. [α]D28 +142.0° (c 0.51, MeOH); ESI-MS (m/z) 514 (M+H)+ as C24H39N3O5S2; TOF-ESI-HRMS (M+H)+ calcd for C24H39N3O5S2: 514.2409, found: 514.2411; 1H NMR (400 MHz, methanol-d4) δ 0.89–0.98 (m, 3 H), 1.20 (d, J=7.1 Hz, 3 H), 1.30–1.41 (m, 4 H), 1.80–1.90 (m, 1 H), 1.92–2.00 (m, 1 H), 2.04–2.21 (m, 2 H), 2.17 (s, 3 H), 2.34 (s, 3 H), 2.98 (dd, J=10.6, 4.6 Hz, 1 H), 3.24 (dd, J=8.2, 5.6 Hz, 1 H), 3.53 (dq, J=7.1, 2.8 Hz, 1 H), 3.60 (dd, J=10.3, 3.3 Hz, 1 H), 3.68–3.72 (m, 1 H), 4.10 (dd, J=10.3, 5.6 Hz, 1 H), 4.25 (dd, J=9.9, 2.8 Hz, 1 H), 4.38 (br d, J=9.9 Hz, 1 H), 5.27 (d, J=5.6 Hz, 1 H), 6.62–6.68 (m, 2 H), 7.20–7.26 (m, 2 H).
7(S)-7-(4-Methanesulfonamidophenylthio)-7-deoxylincomycin (34)
To a solution of compound 33 (83.8 mg, 0.16 mmol) in DMF (1 ml) were added triethylamine (0.027 ml, 0.2 mmol) and methanesulfonyl chloride (0.015 ml, 0.2 mmol) and stirred at RT for 20 min. To the mixture was added saturated aqueous NaHCO3 (10 ml) and then extracted with ethyl acetate, dried over MgSO4 and concentrated under reduced pressure. The resulting residue was purified by preparative TLC (CHCl3/CH3OH/28% aq NH4OH=9/2/0.2) to obtain the title compound as a colorless solid (28.0 mg, 29%). [α]D26 +94.2° (c 1.01, MeOH); ESI-MS (m/z) 592 (M+H)+ as C25H41N3O7S3; TOF-ESI-HRMS (M+H)+ calcd for C25H41N3O7S3: 592.2185, found: 592.2180; 1H NMR (400 MHz, methanol-d4) δ 0.89–0.98 (m, 3 H), 1.28 (d, J=7.0 Hz, 3 H), 1.31–1.41 (m, 4 H), 1.78–1.92 (m, 1 H), 1.99 (ddd, J=12.9, 7.9, 5.0 Hz, 1 H), 2.04 (s, 3 H), 2.05–2.11 (m, 1 H), 2.11–2.23 (m, 1 H), 2.38 (s, 3 H), 2.94–3.03 (m, 1 H), 2.97 (s, 3 H), 3.25 (dd, J=8.1, 5.6 Hz, 1 H), 3.58 (dd, J=10.2, 3.2 Hz, 1 H), 3.74 (br d, J=3.2 Hz, 1 H), 3.78 (dq, J=7.0, 2.5 Hz, 1 H), 4.10 (dd, J=10.2, 5.6 Hz, 1 H), 4.31–4.36 (m, 1 H), 4.38 (dd, J=9.7, 2.5 Hz, 1 H), 5.26 (d, J=5.6 Hz, 1 H), 7.18–7.25 (m, 2 H), 7.40–7.48 (m, 2 H).
7(S)-7-(5-(2-Aminoacetamido)-1,3,4-thiadiazol-2-ylthio)-7-deoxylincomycin(36)
To a solution of compound 1 (240 mg, 0.39 mmol) in THF (5 ml) at 0 °C were added triphenylphosphine (150 mg, 0.57 mmol), diethylazodicarboxylate (0.1 ml, 0.55 mmol) and t-butyl (2-((5-mercapto-1,3,4-thiadiazol-2-yl)amino)-2-oxoethyl)carbamate (150 mg, 0.52 mmol) and stirred RT for 17 h. The mixture was concentrated under reduced pressure, and the resulting residue (compound 35) in 90% aqueous trifluoroacetic acid (5 ml) was stirred at RT for 30 min. The mixture was concentrated under reduced pressure. The resulting residue was purified by preparative reverse-phase column chromatography (YMC triart C18, 20 × 250 mm2, RT, 18.9 ml min−1, 0.1% aq TFA/CH3CN=85/15). This trifluoroacetate was desalted by preparative reverse-phase column chromatography (YMC triart C18, 20 × 250 mm2, RT, 18.9 ml min−1, H2O/MeOH=100/0 (15 min)→0/100 (15–40 min)) to obtain the highly purified title compound as a colorless solid (54.0 mg, 24%). [α]D25 +100.4° (c 0.29, MeOH); ESI-MS (m/z) 578 (M+H)+ as C22H38N6O6S3; TOF-ESI-HRMS (M+H)+ calcd for C22H38N6O6S3: 579.2093, found: 579.2095; 1H NMR (400 MHz, methanol-d4) δ 0.86–0.97 (m, 3 H), 1.29–1.39 (m, 4 H), 1.42 (d, J=7.0 Hz, 3 H), 1.77–1.87 (m, 1 H), 1.93–2.09 (m, 2 H), 2.06 (s, 3 H), 2.13–2.25 (m, 1 H), 2.32 (s, 3 H), 2.98 (dd, J=10.5, 5.0 Hz, 1 H), 3.26 (dd, J=8.5, 6.2 Hz, 1 H), 3.57 (dd, J=10.2, 3.2 Hz, 1 H), 3.54–3.62 (m, 2 H), 3.78 (br dd, J=3.2, 0.5 Hz, 1 H), 4.08 (dq, J=7.0, 2.8 Hz, 1 H), 4.10 (dd, J=10.2, 5.6 Hz, 1 H), 4.40 (br dd, J=9.8, 0.5 Hz, 1 H), 4.47 (dd, J=9.8, 2.8 Hz, 1 H), 5.26 (d, J=5.6 Hz, 1 H).
7(S)-7-(5-Amino-1,3,4-thiadiazol-2-ylthio)-7-deoxy-2,3,4-tris-O-(trimethylsilyl)lincomycin (37)
To a solution of compound 21 (403 mg, 0.77 mmol) in pyridine (8 ml) were added trimethylchlorosilane (0.61 ml, 4.8 mmol) and hexamethyldisilazane (1.05 ml, 5.0 mmol) and stirred at RT for 2.5 h, and then it was concentrated under reduced pressure. The residue was diluted with saturated aqueous NH4Cl and extracted with ethyl acetate, washed with brine, dried over MgSO4 and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (hexane/ethyl acetate=1/1) to obtain the title compound as a colorless solid (467 mg, 82%). ESI-MS (m/z) 738 (M+H)+ as C29H59N5O5S3Si3; 1H NMR (400 MHz, chloroform-d) δ 0.13 (s, 18H), 0.17 (s, 9 H), 0.79–0.97 (m, 3 H), 1.15–1.37 (m, 4 H), 1.42 (d, J=6.9 Hz, 3 H), 1.71–1.90 (m, 2 H), 1.91–2.06 (m, 2 H), 2.08 (s, 3 H), 2.40 (s, 3 H), 2.99 (dd, J=10.7, 3.6 Hz, 1 H), 3.12–3.23 (m, 1 H), 3.61 (dd, J=9.6, 2.5 Hz, 1 H), 3.74 (br d, J=2.2 Hz, 1 H), 4.04–4.24 (m, 3 H), 4.60–4.70 (m, 1 H), 5.23 (d, J=5.5 Hz, 1 H), 5.50 (s, 2 H), 7.59 (d, J=10.7 Hz, 1 H).
7(S)-7-Deoxy-7-(5-(2-methoxyacetamido)-1,3,4-thiadiazol-2-ylthio)lincomycin (38)
To a solution of compound 37 (104 mg, 0.14 mmol) in THF (3 ml) were added triethylamine (0.06 ml, 0.43 mmol) and 2-methoxyacetyl chloride (0.02 ml, 0.22 mmol) and stirred at 0 °C for 2 h. The mixture was diluted with saturated aqueous NH4Cl and extracted with ethyl acetate, washed with brine, dried over MgSO4 and concentrated under reduced pressure. The resulting residue was purified by preparative TLC (CHCl3/CH3OH=19/1) to obtain 7(S)-7-deoxy-7-(5-(2-methoxyacetamido)-1,3,4-thiadiazol-2-ylthio)-2,3,4-tris-O-(trimethylsilyl)lincomycin as a colorless solid (109.8 mg, 96%). 7(S)-7-Deoxy-7-(5-(2-methoxyacetamido)-1,3,4-thiadiazol-2-ylthio)-2,3,4-tris-O-(trimethylsilyl)lincomycin (109.8 mg, 0.14 mmol) in 1 N HCl (1 ml)–MeOH (1 ml) was stirred at RT for 2 h. The mixture was concentrated under reduced pressure. The resulting residue was dissolved by water and washed with diethyl ether. The mixture was added NaHCO3 (150 mg) and then extracted with ethyl acetate, dried over MgSO4 and concentrated under reduced pressure. The resulting residue was purified by preparative TLC (CHCl3/CH3OH/28% aq NH4OH=5/1/0.1) to obtain the title compound as a colorless solid (37.8 mg, 47%). [α]D26 +98.3° (c 0.65, MeOH); ESI-MS (m/z) 594 (M+H)+ as C23H39N5O7S3; TOF-ESI-HRMS (M+H)+ calcd for C23H39N5O7S3: 594.2090, found: 594.2095; 1H NMR (400 MHz, methanol-d4) δ 0.88–0.97 (m, 3 H), 1.30–1.41 (m, 4 H), 1.46 (d, J=7.0 Hz, 3 H), 1.82–1.92 (m, 1 H), 1.97–2.08 (m, 1 H), 2.04 (s, 3 H), 2.08–2.15 (m, 1 H), 2.15–2.27 (m, 1 H), 2.39 (s, 3 H), 3.08 (dd, J=10.4, 5.0 Hz, 1 H), 3.25–3.34 (m, 1 H), 3.48 (s, 3 H), 3.57 (dd, J=10.3, 3.2 Hz, 1 H), 3.77–3.81 (m, 1 H), 4.10 (dd, J=10.3, 5.6 Hz, 1 H), 4.16–4.24 (m, 1 H), 4.21 (s, 2 H), 4.39 (br dd, J=9.8, 0.6 Hz, 1 H), 4.53 (dd, J=9.8, 3.1 Hz, 1 H), 5.26 (d, J=5.6 Hz, 1 H).
7-O-Methanesulfonyl-2,3,4-tris-O-(trimethylsilyl)lincomycin (39)
To a solution of compound 1 (5.0 g, 8.0 mmol) in chloroform (25 ml) were added triethylamine (2.79 ml, 20.1 mmol) and methanesulfonyl chloride (1.24 ml, 16.1 mmol) and stirred at RT for 3 h. The mixture was dissolved by chloroform (60 ml), washed with saturated aqueous NaHCO3 (50 ml), dried over MgSO4 and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (hexane/ethyl acetate=3/1) to obtain the title compound as a colorless solid (5.50 g, 98%). ESI-MS (m/z) 701 (M+H)+ as C28H60N2O8S2Si3; 1H NMR (400 MHz, chloroform-d) δ 0.13 (s, 9 H), 0.14 (s, 9 H), 0.17 (s, 9 H), 0.89 (br t, J=6.9 Hz, 3 H), 1.21–1.36 (m, 4 H), 1.40 (d, J=6.6 Hz, 3 H), 1.79–1.89 (m, 1 H), 1.92–2.09 (m, 3 H), 2.11 (s, 3 H), 2.40 (s, 3 H), 2.99 (dd, J=10.7, 3.7 Hz, 1 H), 3.09 (s, 3 H), 3.14–3.21 (m, 1 H), 3.52 (dd, J=9.5, 2.4 Hz, 1 H), 3.75 (br d, J=2.4 Hz, 1 H), 3.90 (d, J=9.7 Hz, 1 H), 4.15 (dd, J=9.5, 5.6 Hz, 1 H), 4.70–4.78 (m, 1 H), 5.09–5.15 (m, 1 H), 5.16 (d, J=5.6 Hz, 1 H), 7.61 (d, J=10.7 Hz, 1 H).
7(S)-7-Deoxy-7-(4-methoxycarbonylphenylthio)lincomycin (40)
To a solution of compound 39 (100 mg, 0.14 mmol) in DMF (1.0 ml) were added K2CO3 (59.8 mg, 0.43 mmol) and methyl 4-mercaptobenzoate (48.9 mg, 0.29 mmol) and stirred at 80 °C for 3 h. The mixture was cooled down to RT, diluted with 1 N HCl (2 ml)–MeOH (1 ml), stirred at RT for 2 h and concentrated under reduced pressure. The resulting residue was dissolved by water, washed with diethyl ether. To the mixture was added NaHCO3 (150 mg) and then extracted with ethyl acetate, washed with water, dried over MgSO4 and concentrated under reduced pressure. The resulting residue was purified by preparative TLC (CHCl3/CH3OH/28% aq NH4OH=5/1/0.1) to obtain the title compound as a colorless solid (58.0 mg, 73%). [α]D24 +84.6° (c 0.97, MeOH); ESI-MS (m/z) 557 (M+H)+ as C26H40N2O7S2; TOF-ESI-HRMS (M+H)+ calcd for C26H40N2O7S2: 557.2355, found: 557.2359; 1H NMR (400 MHz, methanol-d4) δ 0.88–0.96 (m, 3 H), 1.29–1.37 (m, 4 H), 1.40 (d, J=6.8 Hz, 3 H), 1.79–1.91 (m, 1 H), 1.84 (s, 3 H), 1.96–2.05 (m, 1 H), 2.05–2.12 (m, 1 H), 2.12–2.25 (m, 1 H), 2.40 (s, 3 H), 3.00 (dd, J=10.6, 4.8 Hz, 1 H), 3.24 (dd, J=8.2, 5.7 Hz, 1 H), 3.58 (dd, J=10.2, 3.2 Hz, 1 H), 3.78 (br dd, J=3.2, 0.7 Hz,1 H), 3.89 (s, 3 H), 4.03 (dq, J=6.8, 2.8 Hz,1 H), 4.10 (dd, J=10.2, 5.6 Hz, 1 H), 4.37 (br dd, J=9.7, 0.7 Hz, 1 H), 4.52 (dd, J=9.7, 2.8 Hz, 1 H), 5.24 (d, J=5.6 Hz, 1 H), 7.42–7.49 (m, 2 H), 7.90–7.97 (m, 2 H).
7(S)-7-Deoxy-7-(3-methoxycarbonylphenylthio)lincomycin (41)
Compound 39 (200 mg, 0.29 mmol) and methyl 3-mercaptobenzoate (95.9 mg, 0.57 mmol) were treated according to the similar procedure as described for the preparation of 40 to afford 41 (101.6 mg, 64%) as a colorless solid. [α]D29 +93.6° (c 2.37, MeOH); ESI-MS (m/z) 557 (M+H)+ as C25H37N3O6S2; TOF-ESI-HRMS (M+H)+ calcd for C25H37N3O6S2: 557.2355, found: 557.2355; 1H NMR (400 MHz, methanol-d4) δ 0.88–0.97 (m, 3 H), 1.29–1.40 (m, 4 H), 1.33 (d, J=7.0 Hz, 3 H), 1.78–1.91 (m, 1 H), 1.95–2.04 (m, 1 H), 1.97 (s, 3 H), 2.04–2.11 (m, 1 H), 2.11–2.23 (m, 1 H), 2.39 (s, 3 H), 2.99 (dd, J=10.7, 4.7 Hz, 1 H), 3.23 (dd, J=8.2, 5.7 Hz, 1 H), 3.58 (dd, J=10.2, 3.3 Hz, 1 H), 3.74–3.79 (m, 1 H), 3.87–3.96 (m, 1 H), 3.91 (s, 3 H), 4.11 (dd, J=10.2, 5.6 Hz, 1 H), 4.36 (br dd, J=9.7, 0.6 Hz, 1 H), 4.48 (dd, J=9.7, 2.8 Hz, 1 H), 5.27 (d, J=5.6 Hz, 1 H), 7.42–7.49 (m, 1 H), 7.67 (ddd, J=7.8, 1.8, 1.1 Hz, 1 H), 7.87–7.92 (m, 1 H), 8.02–8.06 (m, 1 H).
In vitro antibacterial activity
MIC, μg ml−1 was determined by the agar dilution method, which was described in Clinical and Laboratory Standards Institute (M7-A5 in 2000). Test strains of S. pneumoniae and S. pyogenes were subjected to seed culture using brain heart infusion agar (Becton Dickinson and Company, Tokyo, Japan) and 5% defibrinated horse blood. Test strains of H. influenzae were subjected to seed culture using sensitivity disk agar-N ‘Nissui’ (SDA; Nissui, Tokyo, Japan), 5% defibrinated horse blood, 5 μg ml−1 Hemin and 15 μg ml−1 NAD. A 5-μl portion of cell suspension of the test strains having about 106 CFU per ml was inoculated into SDA supplemented with 5% defibrinated horse blood, 5 μg ml−1 Hemin and 15 μg ml−1 NAD and incubated at 37 °C for 18–22 h. Then MIC was measured.

Synthesis of 7(S)-7-arylthio-7-deoxylincomycin derivatives. Conditions: a, TMSCl, HMDS, Py, r.t., 2 h; b, 80% AcOH, MeOH, r.t., 16 h; Method A: c, PPh3, DEAD, the corresponding HS-Ar, THF or toluene, 0 °C to 50 °C, 16–18 h, d, 1–2 N HCl, MeOH, r.t., 30 min; Method B: c, PBu3, DEAD, the corresponding Ar-S-S-Ar, THF, 0 °C to r.t., 19–25 h; d, 2 N HCl, r.t., 0.5–3 h; Method C: c, PPh3, DEAD, tert-butyl 5-mercapto-1,3,4-thiadiazol-2-ylcarbamate or tert-butyl 5-mercapto-1,3,4-thiadiazol-2-yl(methyl)carbamate, THF, 0 °C to r.t., 17 h, d, TFA, r.t., 60 min; e, NaOMe, allyl iodide, MeOH, r.t.; f, SnCl2·H2O, NaBH4, EtOH, r.t., 3 h; g, 6-aminobenzo[d]thiazole-2-thiol, K2CO3, DMF, 100 °C, 16 h; h, SnCl2·H2O, NaBH4, EtOH, r.t., 3 h; i, MsCl, TEA, DMF, r.t., 20 min; j, PPh3, DEAD, tert-butyl 2-(5-mercapto-1,3,4-thiadiazol-2-ylamino)-2-oxoethylcarbamate, THF, 0 °C to r.t., 17 h, k, TFA, r.t., 0.5 h; l, TMSCl, HMDS, Py, r.t., 2.5 h; m, 2-methoxyacetyl chloride, TEA, THF, 0 °C, 2 h; n, 1 N HCl, MeOH, r.t., 2 h; o, MsCl, TEA, CHCl3, r.t., 3 h; p, the corresponding HS-Ar, K2CO3, DMF, 80 °C, 3–16 h, q, 1 N HCl, MeOH, r.t., 0.5–2 h.
References
Reinert, R. R., van der Linden, M. & Al-Lahham, A. Molecular characterization of the first telithromycin-resistant Streptococcus pneumoniae isolate in Germany. Antimicrob. Agents Chemother. 49, 3520–3522 (2005).
Kim, S. H. et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob. Agents Chemother. 56, 1418–1426 (2012).
Ajito, K., Miura, T., Furuuchi, T. & Tamura, A. Sixteen-membered macrolides: chemical modifications and future applications. Heterocycles 89, 281–352 (2014).
Schlünzen, F. et al. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature 413, 814–821 (2001).
Poehlsgaard, J. & Douthwaite, S. The macrolide binding site on the bacterial ribosome. Curr. Drug Targets Infect. Disord. 2, 67–78 (2002).
Tu, D., Blaha, G., Moore, P. B. & Steitz, T. A. Structures of MLSBK antibiotics bound to mutated large ribosomal subunits provide a structural explanation for resistance. Cell 121, 257–270 (2005).
Welsblum, B. Erythromycin resisrance by ribosome modification. Antimicrob. Agents Chemother. 39, 577–585 (1995).
Vester, B. & Douthwaite, S. Macrolide resisrance conferred by base substitutions in 23 S rRNA. Antimicrob. Agents Chemother. 45, 1–12 (2001).
O’hara, K. Up- to- date report on the resistance of macrolide antibiotics and future macrolides. Jpn. J. Chemother. 48, 169–190 (2000).
Syrogiannopoulos, G. A. et al. Antimicrobial susceptibility and macrolide resistance inducibility of Streptococcus pneumoniae carrying erm (A), erm (B), or mef (A). Antimicrob. Agents Chemother. 47, 2699–2702 (2003).
Morimoto, S., Takahashi, Y., Watanabe, Y. & Ōmura, S Chemical modification of erythromycins. I. Synthesis and antibacterial activity of 6-O-methylerythromycins A. J. Antibiot. 37, 187–189 (1984).
Slobodan, D. et al. Erythromycin series. Part 13. Synthesis and structure elucidation of 10-dihydro-10-deoxo-11-methyl-11-azaerythromycin A. J. Chem. Res. Synop 1988, 152–153 (1988).
Denis, A. et al. Synthesis and antibacterial activity of HMR 3647 a new ketolide highly potent against erythromycin-resistant and susceptible pathogens. Bioorg. Med. Chem. Lett. 9, 3075–3080 (1999).
Berisio, R. et al. Structural insight into the antibiotic action of telithromycin against resistant mutants. J. Bacteriol. 185, 4276–4279 (2003).
Douthwaite, S., Hansen, L. H. & Mauvais, P. Macrolide-ketolide inhibition of MLS-resistant ribosomes is improved by alternative drug interaction with domain II of 23 S rRNA. Mol. Microbiol. 36, 183–193 (2000).
Hansen, L. H., Mauvais, P. & Douthwaite, S. The macrolide–ketolide antibiotic binding site is formed by structures in domains II and V of 23 S ribosomal RNA. Mol. Microbiol. 31, 623–631 (1999).
Xiong, L., Shah, S., Mauvais, P. & Mankin, A. S. A ketolide resistance mutation in domain II of 23 S rRNA reveals the proximity of hairpin 35 to the peptidyl transferase centre. Mol. Microbiol. 31, 633–639 (1999).
Clay, K. D. et al. Severe hepatotoxicity of telithromycin: three case reports and literature review. Ann. Intern. Med. 144, 415–420 (2006).
Ross, D. B. The FDA and the case of ketek. N. Engl. J. Med. 356, 1601–1604 (2007).
Gleason, P. P., Walters, C., Heaton, A. H. & Schafer, J. A. Telithromycin: the perils of hasty adoption and persistence of off-label prescribing. J. Manag. Care Pharm. 13, 20–25 (2007).
Department of Health and Human Services. Telithromycin (marketed as ketek) information. Available at http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm107824.htm.
Mason, D. J., Dietz, A. & Deboer, C. Lincomycin, a new antibiotic I. Discovery and biological properties. Antimicrob. Agents Chemother. 554–559 (1962).
Magerlein, B. J. & Lincomycin, X. The chemical synthesis of lincomycin. Tetrahedron Lett. 1, 33–36 (1970).
Howarth, G. B., Szarek, W. A. & Jones, J. K. N. The synthesis of lincomycin. J. Chem. Soc. (c) 16, 2218–2224 (1970).
Perlman, D. Structure-Activity Relationships Among the Semisynthetic Antibiotics, (Academic Press, New York, San Francisco, London A Subsidiary of Harcourt Brace Jovanovich, Publishers 600–651 (1977).
Birkenmeyer, R. D. & Kagan, F. Lincomycin. XI. Synthesis and structure of clindamycin. A potent antibacterial agent. J. Med. Chem. 13, 616–619 (1970).
Hoeksema, H Octoses from antibiotics The Upjohn Company, Kalamazoo, Mich., Abstr. Pap. Division of Carbohydrate Chemistry, 149th Meet. Am. Chem. Soc. Detroit, Mich., p. 9C (1965).
Magerlein, B. J., Birkenmeyer, R. D. & Kagan, F. Chemical modification of lincomycin. Antimicrob. Agents Chemother. 727–736 (1966).
Sinkula, A. A., Morozowich, W., Lewis, C. & Mackellar, F. A. Synthesis and bioactivity of lincomycin-7-monoesters. J. Pharm. Sci. 58, 1389–1392 (1969).
Magerlein, B. J. & Kagan, F. Lincomycin. IX. 7-Thiol and thioamido analogs of lincomycin. J. Med. Chem. 12, 974–977 (1969).
Lewis, J. G. et al. Novel Antimicrobial 7-methyl Lincosamides: Prolamide Analogs. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Poster F-1388. (Washington, DC, USA, 2004).
Bannister, B. Modifications of lincomycin involving the carbohydrate portion. Part III. The 7-O-methyl and 6-de-(1-hydroxyethyl) analogues. J. Chem. Soc. Perkin Trans. I 16, 1676–1682 (1973).
Bannister, B. Modifications of lincomycin involving the carbohydrate portion. Part IV. (7S)-7-alkoxy-7-deoxy-analogues. J. Chem. Soc. Perkin Trans. I 360–369 (1974).
Bannister, B. & Mydlow, P. K. The S-alkylation of sulphides by an activated carbohydrate epimine under acidic catalysis: The formation of α-acetamido-sulphides. Part 5. The introduction of functionality into the sulphide substituent. J. Chem. Res. (S) 1989, 90–91 (1989).
Bannister, B. The S-alkylation of sulphides by an activated carbohydrate epimine under acidic catalysis: The formation of α-acetamido-sulphides. Part 4. Reaction with dithioacetals and monothioacetals. J. Chem. Soc., Perkin. Trans. I 1980, 540–552 (1980).
Bannister, B. (7S)-7-deoxy-7-substituted-alkylthio-lincomycin. S-Alkylation of sulphides by an activated epimine under acidic catalysis: formation of α-acetamido-sulphides. Tetrahedron 40, 1633–1660 (1984).
Bannister, B. (The Upjohn company). Derivatives of lincomycin and its analogs and process. US Patent US3915954 A, (1973).
Bannister, B. (The Upjohn company). Derivatives of lincomycin and its analogs and process. Canadian Patent CA-971956 A1 (1972).
Sztaricskai, F. et al. Semisynthetic modification of antibiotic lincomycin. J. Antibiot. 49, 941–943 (1996).
Bannister, B. Modifications of lincomycin involving the carbohydrate portion. Part I. The 2-O-methyl and 2-deoxy-analogues. J. Chem. Soc., Parkin Trans. I 1972, 3025–3030 (1972).
Houtman, R. L. & Mich, P. (The Upjohn company). Trimethylsilyl ethers of lincomycin and its compounds. US Patent US3418414 (1966).
Umemura, E. et al. Synthesis of novel lincomycin derivatives and their in vitro antibacterial activities. J. Antibiot. 66, 195–198 (2013).
Umemura, E. et alLincomycin derivative and antimicrobial agent containing the same as active ingredient. WO 2007/066805 A1 (2007).
Acknowledgements
We thank Mr A Tamura, Dr E Shitara, Dr T Okutomi, Dr T Yoshida, and Mr M Yamamoto for encouragement and valuable discussion. We are grateful to Professor Emeritus Dr M Konno for supervision through our in-house drug discovery program in lincomycin field. We also thank Ms M Ishii for direction in intellectual properties; Mr T Watanabe for contribution toward computational chemistry; Ms S Miki, Ms T Miyara and Ms K Kaneda for analytical and synthetic chemistry; Mr Y Takayama, Ms Y Inoue, Mr T Matsuhira and Ms K Yamada for biological studies; and Ms M Takagi for English manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wakiyama, Y., Kumura, K., Umemura, E. et al. Synthesis and structure–activity relationships of novel lincomycin derivatives. Part 1. Newly generated antibacterial activities against Gram-positive bacteria with erm gene by C-7 modification. J Antibiot 69, 368–380 (2016). https://doi.org/10.1038/ja.2015.119
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2015.119
This article is cited by
-
Synthesis and SARs of novel lincomycin derivatives Part 5: optimization of lincomycin analogs exhibiting potent antibacterial activities by chemical modification at the 6- and 7-positions
The Journal of Antibiotics (2018)
-
Synthesis and antibacterial activity of novel lincomycin derivatives. III. Optimization of a phenyl thiadiazole moiety
The Journal of Antibiotics (2018)
-
Synthesis and antibacterial activity of novel lincomycin derivatives. IV. Optimization of an N-6 substituent
The Journal of Antibiotics (2017)
-
Synthesis and structure–activity relationships of novel lincomycin derivatives. Part 4: synthesis of novel lincomycin analogs modified at the 6- and 7-positions and their potent antibacterial activities
The Journal of Antibiotics (2017)
-
Synthesis and structure–activity relationships of novel lincomycin derivatives part 3: discovery of the 4-(pyrimidin-5-yl)phenyl group in synthesis of 7(S)-thiolincomycin analogs
The Journal of Antibiotics (2017)