Abstract
A new derivative of amphotericin B named amphotericin B(5) has been obtained under the following condition: amphotericin B powder was first dissolved in N,N-dimethylformamide at room temperature to obtain a solution of concentration of 2.0 mg ml−1. Then, the solution was kept at 60 °C in an electric-heated thermostatic water bath for 48–60 h for the purpose of accelerating the degradation. Amphotericin B(5) was isolated by preparative HPLC. Its structure has been identified by means of UV, NMR and LCMS-IT-TOF as (1R, 3S, 5R, 6R, 9R, 11R, 15S, 16R, 17R, 18S, 19E, 21E, 23E, 25E, 27E, 29E, 31E, 33R, 35S, 36R, 37S)-33-[3-form-amide-3,6-dideoxy-β--mannopy-ran-osyl)oxy]-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-di-oxabicyclo [33.3.1] nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylic acid named according to the International Union of Pure and Applied Chemistry. The activity of amphotericin B(5) was tested against Saccharomyces cerevisiae based on microbiological potency using cylinder-plate method, and the toxicities of amphotericin B and amphotericin B(5) were determined simultaneously in zebrafish embryo. The results showed that the toxicity of amphotericin B(5), as revealed by its LD50 value, was lower than amphotericin B.
Similar content being viewed by others
Introduction
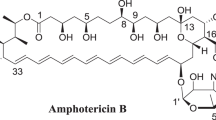
Amphotericin B is an important antifungal antibiotic referred to as a ‘golden standard’ due to its high and broad spectrum of activity.1, 2 It was first isolated from Streptomyces nodosus in 1955 and has been used in the clinic since 1960 to treat a large variety of systemic fungal infections.3, 4 Amphotericin B is a macrocyclic lactone composed of a hydrophilic polyhydroxyl chain on one side and a lipophilic polyene hydrocarbon chain on the other side. Its molecular formula is C47H73NO17, and its chemical structure is shown in Figure 1. To date, amphotericin B remains one of the most potent agents in curing severe and refractory systemic fungal infection among antifungal agents developed. Polyene antifungal antibiotics, an antibiotic family of amphotericin B, selectively associate with ergosterol, which is located in fungal cell membranes, to form transmembrane channels. As a result, cellular components such as potassium and magnesium ions, glucose, and amino acids pass through the channels.5, 6, 7 Amphotericin B, however, has serious side effects such as nephrotoxicity.8, 9 Several new formulations have been developed recently aiming at overcoming its side effects, such as amphotericin B lipid complex, nanoparticles and so on,10, 11 by which the side effects of amphotericin B can be alleviated. According to our experience, the toxicity of antibiotics can be positively correlated with its impurities, which inspired us to study the possibility to reduce toxicity and improve activity by controlling the amount of impurities. In our laboratory, five newly generated compounds under different conditions, named as amphotericin B (1), (2), (3), (5), (7), respectively, have been preliminarily studied. The structure of amphotericin B(2) has been identified.12 In this paper, we report the isolation and structure determination of amphotericin B(5), as well as its primary evaluation of the activity and toxicity.
Results and Discussion
The chemical structure of amphotericin B can be determined by using X-ray diffraction.13 Similarly, due to its complexness, the chemical structure of amphotericin B(5) in this work was analyzed by combining a variety of spectrometric methods, as well as structural comparison with amphotericin B.
UV-Visible
The characteristic UV absorption spectrum of amphotericin B was extremely similar to that of amphotericin B(5). Particularly, there were three absorption peaks at 364.1, 383.3, 407.5 nm and 364.2, 384.3, 407.2 nm in the spectra of amphotericin B and amphotericin B(5), respectively, indicating that the parent conjugated long-chain structure in each component was almost identical.
LCMS-IT-TOF
Amphotericin B and amphotericin B(5) were analyzed using both AB-QTRAP-3200 mass spectrometry (Applied Biosystems, Foster City, CA, USA) and Shimadzu LCMS-IT-TOF mass spectrometry (Shimadzu Corporation, Kyoto, Japan) with identical results being obtained using both systems. The MS data obtained from LCMS-IT-TOF were adopted for structure analysis, for LCMS-IT-TOF mass spectrometry, compared with AB-QTRAP-3200, can determine multi-level decomposition of selected ion peaks, therefore, it is more reliable for resolving the molecular structure and fragmentation pathway of every ion peak. The molecular formula and structure of amphotericin B(5) were proposed based on a comparison of MS data between amphotericin B and amphotericin B(5). The formula of amphotericin B(5) was rationalized to be C48H73NO18 with a molecular weight of 951. The fragmentation information is summarized in Table 1 and the fragmentation pathway is shown accordingly in Figure 2.
As shown in Table 1, the m/z maximum values of amphotericin B(5) of MS1 scan in the positive and negative ion mode were m/z 974.4625 and 950.4698, respectively, and the corresponding difference of positive mode and negative mode was 24, indicating that the former ion peak corresponds to [M+Na]+, whereas the latter denotes [M−H]− ion peak. The molecular weight of amphotericin B(5) can be accordingly determined as 951 based on the MS1 scan result. Particularly, only the MS1 scan results of amphotericin B and amphotericin B(5) were different, whereas their MS2 and MS3 scan results were the same. In the MS2 and MS3 scan, both amphotericin B and amphotericin B(5) produced the same cleavage fragment peaks by the same fragmentation pathway. Specifically, in the MS1 scan, amphotericin B and amphotericin B(5) both formed [M+Na]+ peak ([amphotericin B+Na]+ m/z 946.4657 and [amphotericin B(5)+Na]+ m/z 974.4625). They both lost a sugar chain in the MS2 scan and therefore produced the same ion peak at m/z 783.3810, and subsequently produced the same ion peak at m/z 721.3800 through losing a CO2 and a H2O molecule. It can therefore be inferred that the reaction must occur within the sugar chain (Figure 3), for the sugar chain that was fragmented in the MS2 scan as supported by the same fragment peaks in both MS2 and MS3 scan by amphotericin B and amphotericin B(5). Moreover, because amphotericin B was dissolved in N,N-dimethylformamide and incubated at 60 °C in a water bath to accelerate the reaction, the ammonolysis reaction is very likely to occur within the sugar chain. The molecular formula of amphotericin B(5) was found to be C48H73NO18.
NMR
The amphotericin B(5) structure has also been further confirmed by conducting 1H-NMR and 13C-NMR experiments. Particularly, according to MS analysis, a newly produced amino acyl-C and active amide hydrogen may exist in the sugar chain of amphotericin B(5). Accordingly, the 1H-NMR and 13C-NMR data of this part could be very important (Table 2).
A new 13C-NMR peak δ 202.3 and two new 1H-NMR peaks δ 7.95 and δ 8.02 can be identified in amphotericin B(5) sample, which are very likely from the new fragment produced during the reaction process. Compared with the 1H-NMR and 13C-NMR data of amphotericin B, it can be concluded that the unique spectral shift of amphotericin B(5) exhibited δ 202.3 in the 13C-NMR and δ 7.95, 8.02 in the 1H-NMR can be attributed to the amide bond newly formed in the amino sugar chain, which is consistent with our previous speculation based on the MS data.
Toxicity in zebrafish embryo
The toxicities of both amphotericin B and pure amphotericin B(5) have been examined in TU type zebrafish embryos. This animal model exhibits several advantages including low husbandry cost, short period14, 15, 16 and so on. The samples in toxic study were divided into several groups, including the wild-type control group, 0.1% N,N-dimethylformamide system control group, as well as a series of amphotericin B and amphotericin B(5) groups diluted in artificially sea water at different concentrations. Each group comprised of 30 zebrafish embryos. The procedure was as follows: the drugs were delivered to the healthy embryos at 6 hpf (hour post fertilization) and the embryos were then cultured at room temperature for 3 days. During this incubation period, the sample solution was changed every day and the morphological change of the zebrafish was also monitored daily. The sample concentration was adjusted accordingly to the experiment result until the LD50 was found. The LD50 for amphotericin B and amphotericin B(5) were found to be around 90 ng ml−1 and 5.0 μg ml−1 in this animal model, respectively, suggesting an obvious lower toxicity of amphotericin B(5).
Activity evaluated by microbiological potency using cylinder-plate method
The activities of amphotericin B and amphotericin B(5) were determined according to the cylinder-plate method in Chinese Pharmacopoeia. Specifically, the two-dose method was used in the study. The high and low dosages were set to be 8.0 and 4.0 U ml−1, respectively, according to the preliminary experimental results. The activity of amphotericin B(5) was evaluated by measuring the size of inhibition zone after 16 and 18 h incubation at 35 °C. The potency value results showed that the activity of amphotericin B(5) was 978.4 U mg−1, very close to that of amphotericin B which was no less than 850.0 U mg−1 specified in Chinese Pharmacopoeia. In addition, to broaden the inhibition zone and shorten the incubation time, the buffer pH value in this study was changed from 10.5 to 7.0, and a certain amount of Tween-80 was also added to the buffer.
Experimental procedure
Instruments and methods
Amphotericin B used in this study was provided by NICPBP (National Institute for the Control of Pharmaceutical and Biological Products) in China. The concentration variations in amphotericin B, as well as its derivative, were monitored and separated using a Shimadzu HPLC and Shimadzu preparative HPLC, respectively. Buchi rotary evaporator (BÜCHI Labortechnik AG, Flawil, Switzerland) was used to remove the organic solvent. 1H-NMR and 13C-NMR spectra were recorded on a Bruker Varian Unity Inova-500 (Bruker, Ettlingen, Germany, 500 MHz) (solvent: chloroform-d). Time-of-fight mass spectrometry data were measured in the Shimadzu Corporation. The toxicity tests were conducted using embryos of TU-type zebrafish. Thirty embryos were used for each treatment group. Olympus electron microscope (Olympus, Tokyo, Japan) was used to examine the morphology of the embryo and take pictures. The Saccharomyces cerevisiae and culture medium used in the activity test were also provided by NICPBP.
Sample preparation
A quantity of 100 mg amphotericin B was dissolved in 50 ml N,N-dimethylformamide to give a resultant solution with a concentration of 2.0 mg ml−1. The solution was then placed into an electric-heated thermostatic water bath and kept at 60 °C for 48–60 h to accelerate any degradation or reactive processes. In the end, the contents of the newly generated derivative and amphotericin B were found to be about 31.2 and 64.6%, respectively, monitored by HPLC (Figure 4) (Column: Phenomenex luna C18 (250 × 4.6 mm, 5 μm) mobile phase/organic phase (methanol/acetonitrile=41:18)-buffer (2.5 mmol l−1 EDTA-2Na)=55–45; flow rate: 1.0 ml min−1; column temperature: 30 °C; wavelength: 407 nm; injection volume: 20 μl.). The derivative was isolated by preparative HPLC (column: shim-pack PREP-ODS 228-00815-91 20 mm × 250 mm, 15 μm) with methanol–water (85:15 in volume) solution as a mobile phase at a flow rate of 11.0 ml min−1 and the injection volume of 1.0 ml. The detection wavelength was 407 nm. The amphotericin B(5) fraction was collected and the organic solvent was removed by rotary evaporator. The residual water phase was further purified using LH-20 gel with water as a mobile phase. The derivative of amphotericin B was obtained after being freeze-dried by a vacuum freeze dryer. The purity of amphotericin B(5) was 96.3% (Figure 5) (The HPLC chromatographic condition was same as Figure 4).
Conclusion
A new amphotericin B derivative named amphotericin B(5) has been identified, whose molecular formula was found to be C48H73NO18 with a molecular weight is 951. The sample containing 96.3% amphotericin B(5) was yellowish-brown powder. The structure of amphotericin B(5) was determined based on a variety of spectrometry methods. Its toxicity was examined in a zebrafish animal model, and ascertained to be obviously lower than that of amphotericin B. Meanwhile, the activity of amphotericin B(5) was found to be 978.4 U mg−1 very close to that of amphotericin B as tested by microbiological potency using cylinder-plate method.
References
Gibbs, W. J., Drew, R. H. & Perfect, J. R. Liposomal amphotericin B: clinical experience and perspectives. Expert. Rev. Anti. Infect. Ther. 3, 167–181 (2005).
Ostrosky-Zeichner, L., Marr, K. A., Rex, J. H. & Cohen, S. H. Amphotericin B: time for a new ‘gold standard’. Clin. Infect. Dis. 37, 415–425 (2003).
Torrado, J. J., Espada, A., Ballesteros, M. P. & Torrado-Santiago, S. Amphotericin B formulations and drug targeting. J. Pharm. Sci. 97, 2405–2425 (2008).
Meyer, M. D. current role of therapy with amphotericin B. Clin. Infect. Dis. 14, s154–s160 (1992).
Bolard, J., Legrand, P., Heitz, F. & Cybulska, B. One-sided action of amphotericin B on cholesterol containing membranes is determined by its self association in the medium. Biochemistry 30, 5707–5715 (1991).
Barwicz, J. & Tancrède, P. The effect of aggregation state of amphotericin B on its interactions with cholesterol or ergosterol containing phosphatidylcholine monolayers. Chem. Phys. Lipids. 85, 145–155 (1997).
Barratt, G. & Bretagne, S. Optimizing efficacy of amphotericin B through nanomodification. Int. J. Nanomed. 2, 301–313 (2007).
Sedlák, M., Pravda, M., Kubicová, L., Mikulcíková, P. & Ventura, K. Synthesis and characterisation of a new pH-sensitive amphotericin B—poly(ethylene glycol)-b-poly(L-lysine) conjugate. Bioorg. Med. Chem. Lett. 17, 2554–2557 (2007).
Tiphine, M., Letscher-Bru, V. & Herbrecht, R. Amphotericin B and its new formulations: pharmacologic characteristics, clinical efficacy, and tolerability. Transpl. Infect. Dis. 1, 273–283 (1999).
Lewis, R. E. et al. Comparative analysis of amphotericin B lipid complex and liposomal amphotericin B kinetics of lung accumulation and fungal clearance in a murine model of acute invasive pulmonary Aspergillosis. Antimicrob. Agents. Chemother. 51, 1253–1258 (2007).
Ng, A. W., Wasan, K. M. & Lopez-Berestein, G. Development of liposomal polyene antibiotics: an historical perspective. J. Pharm. Pharmaceut. Sci. 6, 67–83 (2003).
Li, J., Zhu, H. Q., Li, J. Y., Jin, S. H. & Hu, C. Q. Isolation, structure elucidation and activity of an unknown impurity of amphotericin B. J. Antibiot. 60, 272–276 (2007).
Mechlinski, W., Schaffner, C. P., Gann, P. & Avitabile, G. Structure and absolute configuration of the polyene macrolide antibiotic amphotericin B. Tetrahedron. Lett. 44, 3873–3876 (1970).
Spitsbergen, J. M. & Kent, M. L. The state of the art of the Zebrafish Model for Toxicology and Toxicologic Pathology Research—advantages and current limitations. Toxicol. Pathol. 31, 62–87 (2003).
Berry, J. P., Gantar, M., Gibbs, P. D. & Schmale, M. C. The zebrafish (Danio rerio) embryo as a model system for identification and characterization of developmental toxins from marine and freshwater microalgae. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 145, 61–72 (2007).
Hill, A. J., Teraoka, H., Heideman, W. & Peterson, R. E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 86, 6–19 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, YH., Zhang, JP., Chang, Y. et al. A newly identified derivative of amphotericin B: isolation, structure determination and primary evaluation of the activity and toxicity. J Antibiot 63, 553–557 (2010). https://doi.org/10.1038/ja.2010.80
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2010.80
Keywords
This article is cited by
-
Diversity and bioprospecting of culturable actinomycetes from marine sediment of the Yellow Sea, China
Archives of Microbiology (2015)
-
Simultaneous determination of purity and potency of amphotericin B by HPLC
The Journal of Antibiotics (2011)