Abstract
The occurrence of anaerobic oxidation of methane (AOM) and trace methane oxidation (TMO) was investigated in a freshwater natural gas source. Sediment samples were taken and analyzed for potential electron acceptors coupled to AOM. Long-term incubations with 13C-labeled CH4 (13CH4) and different electron acceptors showed that both AOM and TMO occurred. In most conditions, 13C-labeled CO2 (13CO2) simultaneously increased with methane formation, which is typical for TMO. In the presence of nitrate, neither methane formation nor methane oxidation occurred. Net AOM was measured only with sulfate as electron acceptor. Here, sulfide production occurred simultaneously with 13CO2 production and no methanogenesis occurred, excluding TMO as a possible source for 13CO2 production from 13CH4. Archaeal 16S rRNA gene analysis showed the highest presence of ANME-2a/b (ANaerobic MEthane oxidizing archaea) and AAA (AOM Associated Archaea) sequences in the incubations with methane and sulfate as compared with only methane addition. Higher abundance of ANME-2a/b in incubations with methane and sulfate as compared with only sulfate addition was shown by qPCR analysis. Bacterial 16S rRNA gene analysis showed the presence of sulfate-reducing bacteria belonging to SEEP-SRB1. This is the first report that explicitly shows that AOM is associated with sulfate reduction in an enrichment culture of ANME-2a/b and AAA methanotrophs and SEEP-SRB1 sulfate reducers from a low-saline environment.
Similar content being viewed by others
Introduction
Anaerobic methane oxidation (AOM) coupled to sulfate reduction (SR) was first discovered to occur in marine sediments (Martens and Berner, 1974; Reeburgh, 1976). The process was found to be catalyzed by communities of anaerobic methanotrophic archaea (ANME) and sulfate-reducing bacteria (SRB) of the Deltaproteobacteria (Hinrichs et al., 1999; Boetius et al., 2000; Orphan et al., 2001a, 2001b). More recently, AOM was also reported to be coupled to other electron acceptors besides sulfate. In freshwater environments, AOM was coupled to the reduction of nitrate and nitrite (Raghoebarsing et al., 2006; Ettwig et al., 2008, 2009; Hu et al., 2009; Deutzmann and Schink 2011; Haroon et al., 2013). Microbial methane oxidation with iron and/or manganese reduction was described in marine sediments (Beal et al., 2009; Riedinger et al., 2014), brackish sediments (Egger et al., 2015), a terrestrial mud volcano (Chang et al., 2012) and also in freshwater environments (Crowe et al., 2011; Sivan et al., 2011; Amos et al., 2012). Recently, humic acids (HAs) were also hypothesized to act as electron acceptor for AOM (Gupta et al., 2013). AOM coupled to SR in freshwater is likely limited by the low-sulfate concentrations, which are around 10–500 μM (Holmer and Storkholm, 2001).
Sulfate-dependent AOM has been observed in freshwater systems, but the involvement of other electron acceptors could not be excluded. Moreover, the responsible microorganisms were either not analyzed nor conclusively identified (Grossman et al., 2002; van Breukelen and Griffioen, 2004; Eller et al., 2005; Schubert et al., 2011; Segarra et al., 2015). ANME-1-related archaea have been found in a terrestrial subsurface (Takeuchi et al., 2011), but 13C-labeled carbon dioxide (13CO2) formation from 13C-labeled methane (13CH4) also occurred in control incubation where no electron acceptor was added. This was also the case in other incubation studies (Beal et al., 2009; Sivan et al., 2011; Egger et al., 2015). These observations make it difficult to link ongoing methane oxidation to a particular electron acceptor. Moreover, 13CO2 can also be produced during methanogenesis in a process called trace methane oxidation (TMO) (Zehnder and Brock, 1979). TMO was demonstrated to occur in pure cultures of different methanogens (Zehnder and Brock, 1979; Harder, 1997; Moran et al., 2005, 2007), in granular sludge (Zehnder and Brock 1980; Harder 1997; Meulepas et al., 2010) and in freshwater and terrestrial environments (Zehnder and Brock, 1980; Blazewicz et al., 2012). Differentiation between AOM and TMO is difficult for several reasons: (a) both processes can produce 13CO2 at comparable rates; (b) at elevated methane partial pressure, TMO rates increase (Zehnder and Brock, 1980; Smemo and Yavitt, 2007) while methanogenesis is repressed, which favors SR (Meulepas et al., 2010); and (c) ferrous sulfate addition may result in enhanced TMO rates (Zehnder and Brock, 1980). This means that with elevated 13CH4 partial pressure and presence of sulfate, an increase in 13CO2 and sulfide production cannot be taken as evidence for sulfate-dependent AOM unless net methane consumption is demonstrated. Moreover, although there is convincing evidence that anaerobic methane oxidizing archaea (ANME) are capable of net AOM, detecting ANME sequences or cells in mixed communities that perform methanogenesis does not prove that AOM takes place, since ANME could perform methanogenesis as well (Lloyd et al., 2011; Bertram et al., 2013) and as a consequence could perform TMO.
In this study we used long-term incubations (>168 days) with samples taken from a freshwater natural gas source with added 13CH4 to investigate the occurrence of both TMO during net methanogenesis and AOM. AOM was distinguished from TMO by simultaneous detection of 13CH4, 12CH4 (produced during methanogenesis) and 13CO2. We investigated the effect of different electron acceptors that possibly might be involved in AOM. Control incubations without addition of methane were carried out to accurately distinguish between net methane oxidation and net methanogenesis. Archaeal community analysis of long-term incubations with methane and sulfate (CH4+SO42−), sulfate only (SO42−-only), and methane only (CH4-only) was performed at 323 days of incubation. Incubations with sulfate and with and without methane were monitored for an extended period of 728 days.
Materials and methods
Sampling
Samples were taken in spring of 2011 from two natural gas sources in Berkhout, Noord-Holland, The Netherlands (52°38′31″N, 4°59′49″E). These gas sources were used for domestic purposes by capturing natural gas from groundwater pockets, using a 30-m long pipe (Figure 1). Different locations were sampled: the effluent of an active gas source (‘tank’) and the sediment of the ditch where the effluent is collected (‘ditch 1’), the sediment of the ditch where the effluent of the storage tank is collected (‘ditch 2’) and from the sediment inside a gas source that was no longer in use (‘tank 2’, not in Figure 1). Sediment samples were collected in nitrogen flushed bottles with an inversed pump. In the laboratory, the gas phase of the bottles was flushed with 100% 5.5 grade methane (99.999%) and stored at 4 °C for ±21 months. All samples were pooled 1:1:1 (v/v/v) in an anaerobic chamber prior to inoculation.
Schematic representation of the system that is used for capturing natural gas at Berkhout, Noord-Holland, The Netherlands (adapted from Bartstra, 2003). A 30-m deep pipe reaches the pressurized groundwater pockets containing natural gas. Degasification occurs at lower pressure inside the gas source tank where the sprinkler facilitates the process. Gas can be transported to the house or to a storage tank floating on the water ditch. Sampling locations were inside an inactive tank (tank 2, not on picture), from the effluent of the active gas source (tank) and the sediment of the ditch where the effluent is collected (ditch 1) and from the sediment of a ditch where the effluent of the storage tank was collected (ditch 2).
Media composition
Media were prepared as described previously (Stams et al., 1993) using 1 ml l−1 of the vitamin stock solution (for composition, see Supplementary Table S1).
Experimental set-up
Fifteen milliliter aliquots of the pooled sediments (0.07 g volatile suspended solids (gVSS)) were incubated in triplicate in bicarbonate-buffered medium (1:1 v/v) with sulfate (20 mM), iron (as ferrihydrite, 10 mM), humic acids (20 g l−1), iron combined with humic acids (iron as ferrihydrite, 10 mM and humic acids 2 g l−1) and nitrate (20 mM). Iron was combined with humic acids to facilitate electron transfer from insoluble iron to soluble humic acids that can act as an electron shuttle (Kappler et al., 2004). A control without electron acceptor was included. All triplicate conditions were tested with and without 13CH4 in the headspace. All experiments were carried out in 60 ml serum bottles closed with butyl rubber stoppers and aluminum caps. After 10 cycles of exchanging the headspace gas with N2, it was changed to N2/CO2 (1:1) to a pressure of 1.5 bar. When 13CH4 was added, N2/CO2 was added to a pressure of 1.3 bar and 99.99% 13CH4 gas (Campro Scientific, Veenendaal, The Netherlands) was added to a final pressure of 1.8 bar. The serum bottles were incubated at 15 °C in the dark.
Preparation of ferrihydrite
Ferrihydrite (simplified as Fe(OH)3) was produced as described for obtaining nanoparticle size (<10 nm) minerals (Schwertmann and Cornell, 1991). After preparing, the mineral was repeatedly washed and centrifuged for three times and subsequently dialyzed to remove electrolytes. The precipitate was then freeze-dried to remove access water and immediately added to the incubations.
Analytical measurements
Nitrate and sulfate were analyzed by an ion chromatography system equipped with an Ionpac AS9-SC column and an ED 40 electrochemical detector (Dionex, Sunnyvale, CA, USA). The system was operated at a column temperature of 35 °C, and a flow rate of 1.2 ml min−1. Eluent consisted of a carbonate/bicarbonate solution (1.8 and 1.7 mM, respectively) in deionized water.
Headspace gas composition was measured on a gas chromatograph-mass spectrometer composed of a Trace GC Ultra (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a Rt-QPLOT column (Restek, Bellefonte, PA, USA), and a DSQ MS (Thermo Fisher Scientific). Helium was used as a carrier gas with a flow rate of 120 ml min−1 and a split ratio of 60. The inlet temperature was 80 °C, the column temperature was set at 40 °C and the ion source temperature at 200 °C. CH4 and CO2 in the headspace were quantified from the peak areas in the gas chromatographs. The fractions of 13CO2, 13CH4 and 12CH4 were derived from the mass spectrum as previously done (Shigematsu et al., 2004). Validation of the method was carried out using standards with a known mixture of 13CO2, 12CO2, 13CH4 and 12CH4. The concentrations of total CO2, total CH4, 13CO2, 12CH4 (produced during methanogenesis in incubations with 13CH4) and 13CH4 were calculated as described previously (Timmers et al., 2015). Headspace CO2 and CH4 after 168 days of incubation was quantified from the peak areas recorded with a CompactGC gas chromatograph (Global Analyser Solutions, Breda, The Netherlands) containing a Carboxen 1010 precolumn, followed by two lines: a Molsieve 5A column (pressure: 200 kPa, split flow: 20 ml min−1, oven temperature: 80 °C and a PDD detector at 110 °C) and a RT-Q-bond column (pressure: 150 kPa, split flow: 10 ml min−1, oven temperature: 80 °C with a TCD detector at 110 °C) with a carrier gas flow of 10 ml min−1.
The concentrations of iron(II) and iron(III) were measured with the ferrozine colorimetric method (Stookey, 1970). Prior to analysis, samples were acidified with 2 M HCl (1:1 v/v) and centrifuged for 5 min at 15 700 r.c.f. to precipitate humic acids. Absorbance at 562 nm was measured in a U-1500 spectrophotometer (Hitachi, Chiyoda, Tokyo, Japan).
Sulfide concentration was measured with the methylene-blue colorimetric method. Samples were directly diluted 1:1 (v/v) in a 5% (w/v) zinc acetate solution to bind all sulfide. Deionized water was added to a volume of 4.45 ml and 500 μl of reagent A (2 g l−1 dimethylparaphenylenediamine and 200 ml l−1 H2SO4) and 50 μl of reagent B (1 g l−1 Fe((NH4)(SO4))2. 12 H2O and 0.2 ml l−1 H2SO4) were added concurrently and mixed immediately. After 10 min, samples were measured with a Spectroquant Multy colorimeter (Merck Millipore, Darmstadt, Germany) at 660 nm.
Inductively coupled plasma-optical emission spectroscopy using a Vista-MPX CCD simultaneous (Varian Inc., Palo Alto, CA, USA) was used to quantify the elemental composition of all samples, as previously done (Hageman et al., 2013). The standard deviation in all measurements was ⩽1.8%.
The pressure of the serum vials was determined using a portable membrane pressure unit GMH 3150 (Greisinger electronic GmbH, Regenstauf, Germany). The pH was checked by pH paper. Conductivity was measured using a standard electrode. The VSS contents were analyzed according to the standard methods (Clesceri et al., 1995).
DNA extraction
Genomic DNA was extracted from samples after 323 days of incubation from the triplicate incubations with methane and sulfate (CH4+SO42−), sulfate only (SO42−-only), and methane only (CH4-only) and from the original sediment (BHori). DNA was extracted in triplicate for every separate incubation using the Fast DNA Kit for Soil (MP Biomedicals, Santa Ana, CA, USA) according to the manufacturer’s protocol with two 45-s beat beating steps using a Fastprep Instrument (MP Biomedicals). Triplicate extracted DNA for every separate incubation was pooled and DNA concentrations were determined with the Qubit 2.0 fluorometer (Thermo Fisher Scientific).
Bacterial community profiling
Extracted DNA was subjected to barcoded amplification of the V1–V2 region of the 16S rRNA gene. A PCR amplification replicate of BHori (BHoriA and BHoriB) was carried out to correct for technical biases. Barcoded amplification was carried out using forward primer 27F-DegS (van den Bogert et al., 2011) that was extended with the titanium adapter A and an eight-base sample specific barcode (Hamady et al., 2008) at the 5′-end, and an equimolar mix of reverse primers 338R-I and 338R-II (Daims et al., 1999) that were appended with the titanium adapter B at the 5′-end. All primers are listed in Supplementary Table S2. PCR amplification was performed in a thermocycler GS0001 (Gene Technologies, Braintree, UK) in a total volume of 100 μl containing 2 μl DNA (20 ng μl−1), 500 nM of barcoded forward primer and reverse primermix (Biolegio BV, Nijmegen, The Netherlands), 2 U of Phusion Hot start II High-Fidelity DNA polymerase (Finnzymes, Vantaa, Finland), 20 μl of 5 × HF buffer, 2 μl PCR grade nucleotide mix (Roche Diagnostics GmbH, Mannheim, Germany) and 65 μl nuclease-free sterile water (Promega Corporation, Madison, WI, USA). PCR amplification conditions consisted of a pre-denaturing step of 3 min at 98 °C followed by 30 cycles of 98 °C for 10 s, 56 °C for 20 s and 72 °C for 20 s. Lastly, a post-elongation step of 10 min at 72 °C was carried out. PCR products were purified using a GeneJet PCR purification kit (Thermo Fisher Scientific) and the concentration was determined using the Qubit 2.0 fluorometer (Thermo Fisher Scientific). All samples for pyrosequencing were mixed in equimolar amounts. Pooled samples were loaded on a 1% (v/v) agarose gel containing 1 × SYBR Safe (Invitrogen, Thermo Fisher Scientific) and bands of approximately 340 bp were excised and purified with the GeneJet Gel Extraction Kit (Thermo Fisher Scientific) using 25 μl elution buffer for collecting the amplified DNA. Mixed samples were quantified using the Qubit 2.0 fluorometer (Thermo Fisher Scientific) and submitted for pyrosequencing on the 454 Life Sciences GS-FLX platform using Titanium sequencing chemistry (GATC Biotech AG, Konstanz, Germany).
Archaea community profiling
Extracted DNA was subjected to barcoded amplification of the 16S rRNA gene. A PCR amplification replicate of BHori (BHoriA and BHoriB) was carried out to correct for technical biases. A method adapted from Jaeggi et al. (2014) was used. Barcoded amplification of 16S rRNA genes was carried out by using forward primer 340F (Gantner et al., 2011) that was extended with the titanium adapter A and a 10-base sample specific barcode at the 5′-end, and reverse primer 1000R (Gantner et al., 2011) that was appended with the titanium adapter B at the 5′-end. All primers are listed in Supplementary Table S2. PCR amplification was performed in a total volume of 50 μl containing 1 μl DNA, 200 nM of each forward and reverse primer (Biolegio BV), 1 U of KOD Hot Start DNA Polymerase (Merck Millipore), 5 μl of 10 × KOD-buffer, 3 μl MgSO4 (25 mM), 5 μl dNTP mix (2 mM each) and 33 μl nuclease free sterile water. PCR amplification conditions were a pre-denaturing step at 95 °C for 2 min followed by 35 cycles of 95 °C for 20 s, 5 °C for 10s, and 70 °C for 15 s. The approximately 660 bp PCR amplicon was subsequently purified using the MSB Spin PCR apace kit (STRATEC Biomedical AG, Birkenfeld, Germany) and the concentration was checked with a Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific). Purified PCR products were mixed in equimolar amounts. The mixed sample was further purified using the Purelink PCR Purification kit (Thermo Fisher Scientific), with high-cutoff binding buffer B3, and submitted for pyrosequencing on the 454 Life Sciences GS-FLX platform using Titanium sequencing chemistry (GATC Biotech AG).
Pyrosequencing analysis
The pyrosequencing data were analyzed with a workflow based on Quantitative Insights Into Microbial Ecology (QIIME) v1.2 (Caporaso et al., 2010), and reads were filtered for chimeric sequences using the USEARCH algorithm. Operational taxonomic unit (OTU) clustering was performed with settings as recommended in the QIIME newsletter of 17 December 2010 (http://qiime.wordpress.com/2010/12/17/new-default-parameters-for-uclust-otu-pickers/) using an identity threshold of 97%. The SILVA reference database was used for taxonomic classification (Quast et al., 2013). After picking representative OTUs, the relative amount of reads of every OTU to the total amount of reads per sample was quantified. Afterwards, the average relative amount of reads per condition from the biological triplicate samples was calculated. For analysis of the original sample BHori, the average of the PCR duplicates (BHoriA and BHoriB) was calculated. Then, the significant differences of every representative OTU between the conditions CH4+SO42− and CH4-only and between CH4+SO42− and SO42−-only were calculated separately, using the Kruskal–Wallis test (P<0.05). For archaea, we then selected only representative OTUs that were significantly higher in conditions with CH4+SO42− as separately compared with CH4-only and SO42−-only. For bacteria, we selected only representative OTUs that were significantly higher in conditions with CH4+SO42− as compared with both CH4-only and SO42−-only.
Quantitative real-time PCR
Extracted genomic DNA was used for qPCR analysis. The DNA was purified with the DNA clean and concentrator-5 kit (Zymo Research, Irvine, CA, USA) and the concentration was determined with the Qubit 2.0 fluorometer (Thermo Fisher Scientific). Amplifications with specific primers for ANME-2a/b were carried out as described previously (Timmers et al., 2015). Quantification was expressed as the total number of 16S rRNA gene copies per gvss extracted from the incubations.
Nucleotide sequences
Nucleotide sequence data reported are available in the DDBJ/EMBL/GenBank databases under the accession numbers LN795911–LN796465 for archaeal sequences and LN796466–LN808676 for bacterial sequences.
Results and discussion
Trace methane oxidation
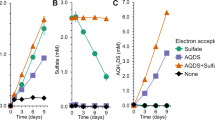
Methane production was observed in most conditions, but was negligible in the presence of sulfate and did not occur in the presence of nitrate (Figure 2). Methane production in conditions with and without added methane showed a similar pattern, but the amount of methane produced was lower in incubations where methane was added (Supplementary Figure S1A). This was probably caused by the increase of TMO due to a higher methane concentration (Zehnder and Brock, 1980; Smemo and Yavitt, 2007). Production of 13CO2 was apparent in all incubations with 13CH4 in the headspace, except in the conditions with nitrate and HAs (Figure 2 and Supplementary Figure S1B). Typical for TMO, 13CO2 simultaneously increased with methane formation in the conditions with ferrihydrite, ferrihydrite + HAs and the control without electron acceptor (Figure 2). The 13CO2 production was not substantially different between ferrihydrite, ferrihydrite + HAs and the control conditions (Wilcoxon rank-sum test, P<0.05; Table 1), indicating that TMO was not influenced by the electron acceptors added. When ferrihydrite was added, the 13CO2 production continued when all iron was reduced to Fe(II) after 300 days, without addition of HAs (Supplementary Figure S2). Iron reduction did occur faster in incubations with ferrihydrite + HAs than in the incubations with only ferrihydrite. The incubations with 20 g l−1 HAs contained an average of 28.8 (±1.0) mM acid soluble Fe(II) after 300 days of incubation and did not show any detectable 13CO2 increase (Figure 2, Supplementary Figure S1B and Table 1). The HAs batch used contained calcium which could scavenge produced CO2 to form calcium carbonate. After acidification of the samples, an increase in total CO2 was observed but the percentage of 13CO2 did not increase. It was reported that reduced methane emission after addition of HAs to peat ecosystems could be caused by increased methane oxidation (Blodau and Deppe, 2012). In contrast, here we observed higher methane production after addition of HAs but no methane oxidation.
Percentage of methane (black lines) and percentage of 13CO2 of total CO2 (gray lines) during 168 days of incubation in bottles with 100% 13CH4 in the headspace and with different electron acceptors. Standard deviations represent triplicate incubations. Note the different scale in the condition with sulfate where one of the triplicates ‘1A-2’ highly increased in 13CO2.
Anaerobic methane oxidation
Only in the incubations with sulfate, an increase in 13CO2 with no increase in 12CH4 was observed (Figure 2). The ratio of methane oxidized per methane produced was only >1 for conditions with sulfate, which is indicative for AOM (Table 1). In previous studies, sulfate addition inhibited methane formation and thus 13CO2 production from TMO in freshwater sludge (Zehnder and Brock, 1980; Meulepas et al., 2010) and in freshwater slurries (Segarra et al., 2013) and only stimulated methane oxidation in brackish water slurries (Segarra et al., 2013). In our study, the pooled inoculum contained an average of around 2 mM sulfate (Supplementary Table S3). All sulfate was reduced after 41 days of incubation and methanogenesis continued in most conditions, which was accompanied by continued 13CO2 production during TMO (Figure 2). Only where sulfate was added, sulfate addition stimulated methane oxidation and repressed methane production, indicating AOM coupled to SR at low salinity. AOM could not be coupled to any other electron acceptor than sulfate. Inductively coupled plasma measurements of all samples prior to mixing showed that only sulfur and iron were present, which in oxidized form could be possible electron acceptors for AOM, whereas the amount of selenium and manganese was not significant (Supplementary Table S4). In incubations with nitrate and humic acids, no 13CO2 was produced.
Reduction of the electron acceptors sulfate, ferrihydrite and nitrate occurred in all conditions with and without addition of methane (Table 2). The reduction rates of sulfate with and without added methane in the first 168 days were similar (two-tailed t-test with unequal variance, P<0.05), which was probably due to endogenous SR masking sulfate-dependent AOM. After 343 days of incubation, the SR rate in incubations with only sulfate had substantially decreased due to endogenous substrate depletion whereas in conditions with methane and sulfate, there was no difference in SR rates. However, in this time period AOM could not be linked to SR and sulfide production as the abundant green sulfur bacteria Chlorobiaceae (Supplementary Figure S3) could have caused the fluctuations in sulfide levels. Growth and activity of Chlorobiaceae explained the green coloration occurring specifically in incubations amended with sulfate, which derived from the bacteriochlorophyll of green sulfur bacteria (Gorlenko, 1970). Green sulfur bacteria are strictly anaerobic autotrophic sulfide oxidizers and have been found to be active when exposed to very little light (Beatty et al., 2005; Manske et al., 2005), which could explain activity even in the dark with limited exposure to light during sampling of our incubations. Their activity probably kept the sulfide concentration low. After maintaining complete darkness in the slurries, the 13CO2 production continued throughout incubation time and free sulfide was eventually measured. In bottle 1A-2 that showed the highest 13CO2 production after 168 days of incubation (Table 1), sulfide production increased simultaneously with 13CO2 production during the last period between 343 and 728 days (Supplementary Figure S4). This shows that at long term, net methane oxidation accompanied sulfide production.
Microbial community profiling
Microbial community profiling was only carried out on triplicates of the conditions CH4+SO42−, CH4-only, SO42−-only, and the original sediment after 323 days of incubation. For all samples that were analyzed, the highest average percentage of 16S rRNA reads for Archaea clustered within the Methanosarcinaceae, Methanoregulaceae, Methanosaetaceae, Methanobacteriaceae, and the Miscellaneous Crenarchaeota Group (MCG) (Supplementary Figure S5). Archaeal OTUs that showed a significantly higher percentage of reads (Kruskal–Wallis, P<0.05) in condition CH4+SO42−, as compared with CH4-only (Figure 3a) and SO42−-only (Figure 3b) make up less than 10% of all reads. In condition CH4+SO42−, ANME-2a/b sequences represented 0.16% of all reads and were much more abundant than in the condition CH4-only. Higher abundance of ANME-2a/b in conditions CH4+SO42− compared with SO42−-only was shown by qPCR analysis (Supplementary Figure S6). This indicates the involvement of ANME-2a/b in AOM coupled to SR, as shown before in marine environments (Orphan et al., 2001a). The ANME-2a/b OTU showed 98% identity with ANME-2a/b from both marine and non-marine environments and do not form a monophyletic cluster with ANME-2a/b found in other low-sulfate environments (Figure 4). A marine enrichment of ANME-2a/b species that share 98% identity was previously shown to be completely inhibited in AOM activity at a salinity of 5‰ (Meulepas et al., 2009), indicating that the ANME-2a/b detected in this study are adapted to low salinity.
Archaeal 16S rDNA pyrosequencing results showing the representative OTUs that were significantly higher in the conditions CH4+SO42− as compared with CH4-only (a) and SO4−2-only (b) (Kruskall–Wallis, P<0.05). Also displayed is the original pooled sample (BHori). Displayed is the average percentage of reads per representative OTU of the three biological triplicates per condition.
Phylogenetic tree of archaeal 16S rRNA gene sequences derived from the SILVA SSU Ref database (release 111). The tree was constructed using the ARB neighbor-joining method with terminal filtering and the Jukes–Cantor correction using almost full-length 16S rRNA sequences. Closed circles represent bootstrap values >70% (1000 replicates). The scale bar represents the percentage of changes per nucleotide position. Short length 16S rRNA sequences (<1000 bp) were afterwards added to the tree using the ARB parsimony method. Color coding represents different sulfate concentrations (mM) of the environments where the sequences were found. Sequences in gray had no clearly reported sulfate data. Sequences found in this study are depicted in black and bold.
A higher percentage of reads was also found for 1 OTU of Methanosarcinales GOM Arc I (OTU 4) in conditions CH4+SO42− compared with both CH4-only and SO42−-only (Figure 3). This GOM Arc I group was previously named ‘ANME-2d’ (Mills et al., 2003) but was renamed to ‘GOM Arc I’ since it was not monophyletic with other ANME-2 subtypes and no AOM activity or aggregation with sulfate reducers had been shown (Lloyd et al., 2006). Recently, the name ANME-2d was re-adopted for a cluster that harbors ‘Ca. Methanoperedens nitroreducens’, which performed AOM coupled to nitrate reduction (Haroon et al., 2013). This cluster was previously identified in a nitrate-dependent AOM enrichment (Raghoebarsing et al., 2006) and was named ‘AOM associated archaea’ (AAA) (Knittel and Boetius, 2009). The GOM Arc I related OTU 4 found in this study was 97% identical to ‘Ca. M. nitroreducens’ and was 99% identical to other AAA members that were proposed to be responsible for freshwater AOM coupled to SR in Lago di Cadagno sediments (Schubert et al., 2011) (Figure 4). The AAA were also found to be abundant in an aquifer where methane and sulfate were present (Flynn et al., 2013). It was already shown that ‘Ca. M. nitroreducens’ uses the complete reverse methanogenesis pathway and it was suggested that the genes for nitrate reduction were obtained from a bacterial donor (Haroon et al., 2013). We did not find nitrate-dependent AOM activity, which leaves open the possibility that the AAA in this study could perform AOM coupled to SR. Sulfate addition in the presence of methane also had a positive effect on other GOM Arc I related OTUs (Figures 3a and 4), which makes a contribution of GoM Arc I to AOM activity likely. The higher percentage of reads of Methanolobus in conditions CH4+SO42− compared with SO42−-only (Figure 3b) implied that methane addition had an effect on Methanolobus abundance. The reason for this effect is unclear, since this genus is known to be able to utilize methylated compounds (Zhang et al., 2008), but not methane. However, Methanolobus was also found in a marine methane-oxidizing bioreactor (Girguis et al., 2003).
Bacterial diversity was high in all samples, with the highest relative number of reads for all samples clustering with the Deltaproteobacteria (Syntrophobacteriacaea and Desulfobacteraceae) and Gammaproteobacteria (Methylococcaceae), Bacteroidetes, Chloroflexi, Firmicutes and Chlorobi (family Chlorobiaceae) (Supplementary Figure S3). Bacterial OTUs that showed a substantially higher percentage of reads (Kruskal–Wallis, P<0.05) in condition CH4+SO42− as compared with both CH4-only and SO42−-only make up less than 0.5% of all reads (Figure 5). These OTUs clustered with the Desulfobacteraceae, Clostridiales and Planctomycetaceae. The OTUs of Desulfobacteraceae belonged to the Sva0081 sediment group, Desulfobacterium spp. and the SEEP-SRB1 cluster. The latter OTU of SEEP-SRB1 (AB630772) was only found in condition CH4+SO42−. However, other SEEP-SRB1 OTUs that were detected did not show a difference in read abundance between the conditions CH4+SO42−, CH4-only and SO42−-only. The SEEP-SRB1 clade has been detected in several marine AOM-mediating environments (Orphan et al., 2001b; Knittel et al., 2003; Lösekann et al., 2007; Pernthaler et al., 2008; Harrison et al., 2009; Yanagawa et al., 2011; Vigneron et al., 2013) and enrichments (Jagersma et al., 2009; Zhang et al., 2011). The SEEP-SRB1 OTUs found in this study clustered in undefined subgroups outside the marine SEEP-SRB1 subgroups that were described previously (Figure 6), of which SEEP-SRB1a was identified as the dominant bacterial partner of ANME-2a/b in marine AOM-mediating enrichments (Schreiber et al., 2010). From the other OTUs that showed a higher percentage of reads in condition CH4+SO42−, little is known about their role in AOM coupled to SR. It has been shown before that different SRB besides SEEP-SRB1 belonging to Desulfobacteraceae form consortia with different ANMEs (Orphan et al., 2002; Vigneron et al., 2013) and even non-SRB were found to aggregate with ANMEs (Pernthaler et al., 2008). We did not find any sequences related to the NC10 phylum of bacteria, harboring the nitrate-dependent methanotrophic bacterium ‘Ca. Methylomirabilis oxyfera’ (Ettwig et al., 2010), and we also did not obtain any PCR product using specific primers for this clade (data not shown), which is in line with the lack of AOM coupled to denitrification.
Bacterial 16S rDNA pyrosequencing results showing the representative OTUs that were significantly higher in the conditions CH4+SO42− as compared with both CH4-only and SO42−-only (Kruskall–Wallis, P<0.05). Also displayed is the original pooled sample (BHori). Displayed is the average percentage of reads per representative OTU of the three biological triplicates per condition.
Phylogenetic tree of bacterial 16S rRNA gene sequences derived from the SILVA SSU Ref database (release 111). The tree was constructed using the ARB neighbor-joining method with terminal filtering and the Jukes–Cantor correction using almost full-length 16S rRNA sequences. Closed circles represent bootstrap values >70% (1000 replicates). The scale bar represents the percentage of changes per nucleotide position. Short length 16S rRNA sequences (<1000 bp) were afterwards added to the tree using the ARB parsimony method. Color coding represents different sulfate concentrations (mM) of the environments where the sequences were found. Sequences in gray had no clearly reported sulfate data. Sequences found in this study are depicted in black and bold.
AOM at low-sulfate concentrations
The sulfate concentration was 0.07 mM in the gas source effluent and about 2 mM in the pooled inoculum (Supplementary Table S3). The measured conductivity and chloride concentration of the gas source effluent and pooled inoculum samples (Supplementary Table S3) indicate a somewhat higher salinity than typical freshwater, but a much lower salinity than typical brackish environments. This could correspond to the historical marine influence of the adjacent lake (Markermeer) that was formed due to dike construction, as described for proximal sites (van Diggelen et al., 2014). In marine environments, the sulfate:chloride ratio is around 1:19. The sulfate:chloride ratio of the lake surface water was around 1:2.6, with 1.7 mM sulfate and 4.4 mM chloride (Supplementary Table S3). Therefore, marine influences cannot explain the relatively high-sulfate concentrations. The sulfate concentration in deeper layers of the gas source could be even higher than measured in the gas source effluent before AOM took place. In marine systems, AOM rates started to be affected below 2–3 mM sulfate (Meulepas et al., 2009; Wegener and Boetius, 2009) but occurred even below 0.5 mM of sulfate (Beal et al., 2011; Yoshinaga et al., 2014). In typical freshwater environments, the sulfate concentration is generally lower than 0.5 mM, making AOM-SR feasible but at low rates. AOM in freshwater was recently shown to be a strong methane sink at sulfate concentrations as low as 1.2–0.1 mM (Segarra et al., 2015). Our finding of AOM activity only in conditions with methane and sulfate, and the enrichment of ANME-2a/b and SEEP-SRB1, suggests that these syntrophic clades are ubiquitously distributed in marine and in low-salinity environments and perform AOM at low-sulfate concentrations.
Accession codes
References
Amos RT, Bekins BA, Cozzarelli IM, Voytek MA, Kirshtein JD, Jones EJ et al. (2012). Evidence for iron-mediated anaerobic methane oxidation in a crude oil-contaminated aquifer. Geobiology 10: 506–517.
Bartstra D . (2003). Gasbronnen in Noord-Holland. Vereniging tot behoud van gasbronnen in Noord-Holland, Middenbeemster, Netherlands ISBN 9789090169125.
Beal EJ, House CH, Orphan VJ . (2009). Manganese- and iron-dependent marine methane oxidation. Science 325: 184–187.
Beal EJ, Claire MW, House CH . (2011). High rates of anaerobic methanotrophy at low sulfate concentrations with implications for past and present methane levels. Geobiology 9: 131–139.
Beatty JT, Overmann J, Lince MT, Manske AK, Lang AS, Blankenship RE et al. (2005). An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent. Proc Natl Acad Sci USA 102: 9306–9310.
Bertram S, Blumenberg M, Michaelis W, Siegert M, Krüger M, Seifert R . (2013). Methanogenic capabilities of ANME-archaea deduced from 13C-labelling approaches. Environ Microbiol 15: 2384–2393.
Blazewicz SJ, Petersen DG, Waldrop MP, Firestone MK . (2012). Anaerobic oxidation of methane in tropical and boreal soils: ecological significance in terrestrial methane cycling. J Geophys Res Biogeosci 117: G02033.
Blodau C, Deppe M . (2012). Humic acid addition lowers methane release in peats of the Mer Bleue bog, Canada. Soil Biol Biochem 52: 96–98.
Boetius A, Ravenschlag K, Schubert CJ, Rickert D, Widdel F, Gieseke A et al. (2000). A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407: 623–626.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336.
Chang YH, Cheng TW, Lai WJ, Tsai WY, Sun CH, Lin LH et al. (2012). Microbial methane cycling in a terrestrial mud volcano in eastern Taiwan. Environ Microbiol 14: 895–908.
Clesceri LS, Greenberg AE, Trussel RR eds. (1995) Standard Methods for the Examination of Water and Wastewater, 19th edn. APHA-AWWA-WEF: Washington, DC, USA.
Crowe SA, Katsev S, Leslie K, Sturm A, Magen C, Nomosatryo S et al. (2011). The methane cycle in ferruginous Lake Matano. Geobiology 9: 61–78.
Daims H, Bruhl A, Amann R, Schleifer KH, Wagner M . (1999). The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22: 434–444.
Deutzmann JS, Schink B . (2011). Anaerobic oxidation of methane in sediments of Lake Constance, an oligotrophic freshwater lake. Appl Environ Microbiol 77: 4429–4436.
Egger M, Rasigraf O, Sapart CJ, Jilbert T, Jetten MSM, Rockmann T et al. (2015). Iron-mediated anaerobic oxidation of methane in brackish coastal sediments. Environ Sci Technol 49: 277–283.
Eller G, Kanel L, Krüger M . (2005). Cooccurrence of aerobic and anaerobic methane oxidation in the water column of Lake Plusssee. Appl Environ Microbiol 71: 8925–8928.
Ettwig KF, Shima S, van de Pas-Schoonen KT, Kahnt J, Medema MH, Op den Camp HJM et al. (2008). Denitrifying bacteria anaerobically oxidize methane in the absence of Archaea. Environ Microbiol 10: 3164–3173.
Ettwig KF, van Alen T, van de Pas-Schoonen KT, Jetten MSM, Strous M . (2009). Enrichment and molecular detection of denitrifying methanotrophic bacteria of the NC10 phylum. Appl Environ Microbiol 75: 3656–3662.
Ettwig KF, Butler MK, Le Paslier D, Pelletier E, Mangenot S, Kuypers MMM et al. (2010). Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464: 543–548.
Flynn TM, Sanford RA, Ryu H, Bethke CM, Levine AD, Ashbolt NJ et al. (2013). Functional microbial diversity explains groundwater chemistry in a pristine aquifer. BMC Microbiol 13: 146.
Gantner S, Andersson AF, Alonso-Saez L, Bertilsson S . (2011). Novel primers for 16S rRNA-based archaeal community analyses in environmental samples. J Microbiol Meth 84: 12–18.
Girguis PR, Orphan VJ, Hallam SJ, DeLong EF . (2003). Growth and methane oxidation rates of anaerobic methanotrophic archaea in a continuous-flow bioreactor. Appl Environ Microbiol 69: 5472–5482.
Gorlenko VM . (1970). A new ohototrophic green sulphur bacterium-Prosthecochloris aestuarii nov. gen. nov. spec. Z Allg Mikrobiol 10: 147–149.
Grossman EL, Cifuentes LA, Cozzarelli IM . (2002). Anaerobic methane oxidation in a landfill-leachate plume. Environ Sci Technol 36: 2436–2442.
Gupta V, Smemo KA, Yavitt J, Fowle DA, Branfireun BA, Basiliko N . (2013). Stable isotopes reveal widespread anaerobic methane oxidation across latitude and peatland type. Environ Sci Technol 47: 8273–8279.
Hageman SPW, van der Weijden RD, Weijma J, Buisman CJN . (2013). Microbiological selenate to selenite conversion for selenium removal. Water Res 47: 2118–2128.
Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R . (2008). Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods 5: 235–237.
Harder J . (1997). Anaerobic methane oxidation by bacteria employing 14C-methane uncontaminated with 14C-carbon monoxide. Mar Geol 137: 13–23.
Haroon MF, Hu SH, Shi Y, Imelfort M, Keller J, Hugenholtz P et al. (2013). Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500: 567–570.
Harrison BK, Zhang H, Berelson W, Orphan VJ . (2009). Variations in archaeal and bacterial diversity associated with the sulfate-methane transition zone in continental margin sediments (Santa Barbara Basin, California). Appl Environ Microbiol 75: 1487–1499.
Hinrichs KU, Hayes JM, Sylva SP, Brewer PG, DeLong EF . (1999). Methane-consuming archaebacteria in marine sediments. Nature 398: 802–805.
Holmer M, Storkholm P . (2001). Sulphate reduction and sulphur cycling in lake sediments: a review. Freshwater Biol 46: 431–451.
Hu SH, Zeng RJ, Burow LC, Lant P, Keller J, Yuan ZG . (2009). Enrichment of denitrifying anaerobic methane oxidizing microorganisms. Environ Microbiol Rep 1: 377–384.
Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A et al. (2014). Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 64: 731–742.
Jagersma GC, Meulepas RJW, Heikamp-de Jong I, Gieteling J, Klimiuk A, Schouten S et al. (2009). Microbial diversity and community structure of a highly active anaerobic methane-oxidizing sulfate-reducing enrichment. Environ Microbiol 11: 3223–3232.
Kappler A, Benz M, Schink B, Brune A . (2004). Electron shuttling via humic acids in microbial iron(III) reduction in freshwater sediment. FEMS Microbiol Ecol 47: 85–92.
Knittel K, Boetius A, Lemke A, Eilers H, Lochte K, Pfannkuche O et al. (2003). Activity, distribution, and diversity of sulfate reducers and other bacteria in sediments above gas hydrate (Cascadia margin, Oregon). Geomicrobiol J 20: 269–294.
Knittel K, Boetius A . (2009). Anaerobic oxidation of methane: progress with an unknown process. Annu Rev Microbiol 63: 311–334.
Lloyd KG, Lapham L, Teske A . (2006). Anaerobic methane-oxidizing community of ANME-1b archaea in hypersaline Gulf of Mexico sediments. Appl Environ Microbiol 72: 7218–7230.
Lloyd KG, Alperin MJ, Teske A . (2011). Environmental evidence for net methane production and oxidation in putative ANaerobic MEthanotrophic (ANME) archaea. Environ Microbiol 13: 2548–2564.
Lösekann T, Knittel K, Nadalig T, Fuchs B, Niemann H, Boetius A et al. (2007). Diversity and abundance of aerobic and anaerobic methane oxidizers at the Haakon Mosby mud volcano, Barents Sea. Appl Environ Microbiol 73: 3348–3362.
Manske AK, Glaeser J, Kuypers MM, Overmann J . (2005). Physiology and phylogeny of green sulfur bacteria forming a monospecific phototrophic assemblage at a depth of 100 meters in the Black Sea. Appl Environ Microbiol 71: 8049–8060.
Martens CS, Berner RA . (1974). Methane production in interstitial waters of sulfate-depleted marine sediments. Science 185: 1167–1169.
Meulepas RJW, Jagersma CG, Khadem AF, Buisman CJN, Stams AJM, Lens PNL . (2009). Effect of environmental conditions on sulfate reduction with methane as electron donor by an Eckernforde Bay enrichment. Environ Sci Technol 43: 6553–6559.
Meulepas RJW, Jagersma CG, Zhang Y, Petrillo M, Cai H, Buisman CJN et al. (2010). Trace methane oxidation and the methane dependency of sulfate reduction in anaerobic granular sludge. FEMS Microbiol Ecol 72: 261–271.
Mills HJ, Hodges C, Wilson K, MacDonald IR, Sobecky PA . (2003). Microbial diversity in sediments associated with surface-breaching gas hydrate mounds in the Gulf of Mexico. FEMS Microbiol Ecol 46: 39–52.
Moran JJ, House CH, Freeman KH, Ferry JG . (2005). Trace methane oxidation studied in several Euryarchaeota under diverse conditions. Archaea 1: 303–309.
Moran JJ, House CH, Thomas B, Freeman KH . (2007). Products of trace methane oxidation during nonmethylotrophic growth by Methanosarcina. J Geophys Res-Biogeo 112: G02011.
Orphan VJ, House CH, Hinrichs KU, McKeegan KD, DeLong EF . (2001a). Methane-consuming archaea revealed by directly coupled isotopic and phylogenetic analysis. Science 293: 484–487.
Orphan VJ, Hinrichs KU, Ussler W, Paull CK, Taylor LT, Sylva SP et al. (2001b). Comparative analysis of methane-oxidizing archaea and sulfate-reducing bacteria in anoxic marine sediments. Appl Environ Microbiol 67: 1922–1934.
Orphan VJ, House CH, Hinrichs KU, McKeegan KD, DeLong EF . (2002). Multiple archaeal groups mediate methane oxidation in anoxic cold seep sediments. P Natl Acad Sci USA 99: 7663–7668.
Pernthaler A, Dekas AE, Brown CT, Goffredi SK, Embaye T, Orphan VJ . (2008). Diverse syntrophic partnerships from-deep-sea methane vents revealed by direct cell capture and metagenomics. P Natl Acad Sci USA 105: 7052–7057.
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41: D590–D596.
Raghoebarsing AA, Pol A, van de Pas-Schoonen KT, Smolders AJP, Ettwig KF, Rijpstra WIC et al. (2006). A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440: 918–921.
Reeburgh WS . (1976). Methane consumption in Cariaco Trench waters and sediments. Earth Planet Sc Lett 28: 337–344.
Riedinger N, Formolo MJ, Lyons TW, Henkel S, Beck A, Kasten S . (2014). An inorganic geochemical argument for coupled anaerobic oxidation of methane and iron reduction in marine sediments. Geobiology 12: 172–181.
Schreiber L, Holler T, Knittel K, Meyerdierks A, Amann R . (2010). Identification of the dominant sulfate-reducing bacterial partner of anaerobic methanotrophs of the ANME-2 clade. Environ Microbiol 12: 2327–2340.
Schubert CJ, Vazquez F, Lösekann-Behrens T, Knittel K, Tonolla M, Boetius A . (2011). Evidence for anaerobic oxidation of methane in sediments of a freshwater system (Lago di Cadagno). FEMS Microbiol Ecol 76: 26–38.
Schwertmann U, Cornell RM . (1991) Ferrihydrite In: Schwertmann U, Cornell RM (eds) Iron Oxides in the Laboratory: Preparation and Characterization. WILEY-VCH Verlag GmbH, pp 103–112.
Segarra KEA, Comerford C, Slaughter J, Joye SB . (2013). Impact of electron acceptor availability on the anaerobic oxidation of methane in coastal freshwater and brackish wetland sediments. Geochim Cosmochim Acta 115: 15–30.
Segarra KEA, Schubotz F, Samarkin V, Yoshinaga MY, Hinrichs KU, Joye SB . (2015). High rates of anaerobic methane oxidation in freshwater wetlands reduce potential atmospheric methane emissions. Nat Commun 6: 7477.
Shigematsu T, Tang YQ, Kobayashi T, Kawaguchi H, Morimura S, Kida K . (2004). Effect of dilution rate on metabolic pathway shift between aceticlastic and nonaceticlastic methanogenesis in chemostat cultivation. Appl Environ Microbiol 70: 4048–4052.
Sivan O, Adler M, Pearson A, Gelman F, Bar-Or I, John SG et al. (2011). Geochemical evidence for iron-mediated anaerobic oxidation of methane. Limnol Oceanogr 56: 1536–1544.
Smemo KA, Yavitt JB . (2007). Evidence for anaerobic CH4 oxidation in freshwater peatlands. Geomicrobiol J 24: 583–597.
Stams AJM, van Dijk JB, Dijkema C, Plugge CM . (1993). Growth of syntrophic propionate-oxidizing bacteria with fumarate in the absence of methanogenic bacteria. Appl Environ Microbiol 59: 1114–1119.
Stookey LL . (1970). Ferrozine - a new spectrophotometric reagent for iron. Anal Chem 42: 779–781.
Takeuchi M, Yoshioka H, Seo Y, Tanabe S, Tamaki H, Kamagata Y et al. (2011). A distinct freshwater-adapted subgroup of ANME-1 dominates active archaeal communities in terrestrial subsurfaces in Japan. Environ Microbiol 13: 3206–3218.
Timmers PHA, Gieteling J, Widjaja-Greefkes HCA, Plugge CM, Stams AJM, Lens PNL et al. (2015). Growth of anaerobic methane oxidizing archaea and sulfate reducing bacteria in a high pressure membrane-capsule bioreactor. Appl Environ Microbiol 81: 1286–1296.
van Breukelen BM, Griffioen J . (2004). Biogeochemical processes at the fringe of a landfill leachate pollution plume: potential for dissolved organic carbon, Fe(II), Mn(II), NH4, and CH4 oxidation. J Contam Hydrol 73: 181–205.
van den Bogert B, de Vos WM, Zoetendal EG, Kleerebezem M . (2011). Microarray analysis and barcoded pyrosequencing provide consistent microbial profiles depending on the source of human intestinal samples. Appl Environ Microbiol 77: 2071–2080.
van Diggelen JMH, Lamers LPM, van Dijk G, Schaafsma MJ, Roelofs JGM, Smolders AJP . (2014). New insights into phosphorous mobilisation from sulphur-rich sediments: time-dependent effects of salinisation. PLOS One 9: e111106.
Vigneron A, Cruaud P, Pignet P, Caprais JC, Gayet N, Cambon-Bonavita MA et al. (2013). Bacterial communities and syntrophic associations involved in anaerobic oxidation of methane process of the Sonora Margin cold seeps, Guaymas Basin. Environ Microbiol 16: 2777–2790.
Wegener G, Boetius A . (2009). An experimental study on short-term changes in the anaerobic oxidation of methane in response to varying methane and sulfate fluxes. Biogeosciences 6: 867–876.
Yanagawa K, Sunamura M, Lever MA, Morono Y, Hiruta A, Ishizaki O et al. (2011). Niche separation of methanotrophic archaea (ANME-1 and-2) in methane-seep sediments of the eastern Japan sea offshore Joetsu. Geomicrobiol J 28: 118–129.
Yoshinaga MY, Holler T, Goldhammer T, Wegener G, Pohlman JW, Brunner B et al. (2014). Carbon isotope equilibration during sulphate-limited anaerobic oxidation of methane. Nat Geosci 7: 190–194.
Zehnder AJB, Brock TD . (1979). Methane formation and methane oxidation by methanogenic bacteria. J Bacteriol 137: 420–432.
Zehnder AJB, Brock TD . (1980). Anaerobic methane oxidation: occurrence and ecology. Appl Environ Microbiol 39: 194–204.
Zhang G, Jiang N, Liu X, Dong X . (2008). Methanogenesis from methanol at low temperatures by a novel psychrophilic methanogen, “Methanolobus psychrophilus”sp. nov., prevalent in Zoige wetland of the Tibetan plateau. Appl Environ Microbiol 74: 6114–6120.
Zhang Y, Maignien L, Zhao X, Wang F, Boon N . (2011). Enrichment of a microbial community performing anaerobic oxidation of methane in a continuous high-pressure bioreactor. BMC Microbiol 11: 137.
Acknowledgements
We thank Douwe Bartstra (Vereniging tot Behoud van de Gasbronnen in Noord-Holland, The Netherlands), Carla Frijters (Paques BV, The Netherlands) and Teun Veuskens (Laboratory of Microbiology, WUR, The Netherlands) for sampling; Martin Meirink (Hoogheemraadschap Hollands Noorderkwartier, The Netherlands) for physico-chemical data; Freek van Sambeek for providing Figure 1; Lennart Kleinjans (Laboratory of Microbiology, WUR, The Netherlands) for help with pyrosequencing analysis, Irene Sánchez-Andrea (Laboratory of Microbiology, WUR, The Netherlands) for proof-reading and Katharina Ettwig (Department of Microbiology, Radboud University Nijmegen, The Netherlands) for providing M. oxyfera DNA. We want to thank all anonymous reviewers for valuable contributions. This research is supported by the Dutch Technology Foundation STW (project 10711), which is part of the Netherlands Organization for Scientific Research (NWO), and which is partly funded by the Ministry of Economic Affairs. Research of AJMS is supported by ERC grant (project 323009) and the Gravitation grant (project 024.002.002) of the Netherlands Ministry of Education, Culture and Science and the Netherlands Science Foundation (NWO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Timmers, P., Suarez-Zuluaga, D., van Rossem, M. et al. Anaerobic oxidation of methane associated with sulfate reduction in a natural freshwater gas source. ISME J 10, 1400–1412 (2016). https://doi.org/10.1038/ismej.2015.213
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2015.213
This article is cited by
-
Microbial life in 25-m-deep boreholes in ancient permafrost illuminated by metagenomics
Environmental Microbiome (2023)
-
Survival strategies of an anoxic microbial ecosystem in Lake Untersee, a potential analog for Enceladus
Scientific Reports (2022)
-
Discovery of a new method to reduce methane emissions from farm dairy effluent
Journal of Soils and Sediments (2021)
-
Characterization of microbial communities in sediments of the South Yellow Sea
Journal of Oceanology and Limnology (2021)
-
Interactions of sulfur and methane-oxidizing bacteria in tropical estuarine sediments
Environmental Monitoring and Assessment (2019)