Abstract
Epithelial surfaces of most animals are colonized by diverse microbial communities. Although it is generally agreed that commensal bacteria can serve beneficial functions, the processes involved are poorly understood. Here we report that in the basal metazoan Hydra, ectodermal epithelial cells are covered with a multilayered glycocalyx that provides a habitat for a distinctive microbial community. Removing this epithelial microbiota results in lethal infection by the filamentous fungus Fusarium sp. Restoring the complex microbiota in gnotobiotic polyps prevents pathogen infection. Although mono-associations with distinct members of the microbiota fail to provide full protection, additive and synergistic interactions of commensal bacteria are contributing to full fungal resistance. Our results highlight the importance of resident microbiota diversity as a protective factor against pathogen infections. Besides revealing insights into the in vivo function of commensal microbes in Hydra, our findings indicate that interactions among commensal bacteria are essential to inhibit pathogen infection.
Similar content being viewed by others
Introduction
In the past decade, it became evident that the epithelia of most animals are associated with complex microbial communities (McFall-Ngai et al., 2013), inhabiting a broad range of body niches like the intestinal tract, oral cavity, skin, body fluids (Human Microbiome Project Consortium, 2012) or even specialized structures like bacteriocytes or cuticular pouches in insects (Douglas and Wilkinson, 1998; Currie et al., 2006). In vertebrates, the gastrointestinal tract is colonized with a dense and diverse microbial community that is an important factor in health and physiology (Lozupone et al., 2012; Sommer and Bäckhed, 2013). The diversity in microbiota composition and habitats is equaled by a broad variety of beneficial functions to the colonized host. The intestinal microbiota can stimulate stem cell turnover (Jones et al., 2013), gut development (Rawls et al., 2004) and facilitate nutrient supply by breakdown of complex carbohydrates or synthesis of essential amino acids (Sandström et al., 2000; Douglas et al., 2001; Yatsunenko et al., 2012). Furthermore, commensal microbes are able to stimulate fundamental aspects of innate and adaptive immunity such as T-cell maturation, production of IgA, mucus secretion and induction of antimicrobial peptides (Dobber et al., 1992; Mazmanian et al., 2005; Weiss et al., 2012).
In 1955, Bohnhoff et al. (1955) already demonstrated that mice with intact endogenous bacterial colonization require 100 000 times higher inocula to establish Salmonella enterica infection compared with streptomycin-treated mice, a mechanism known as ‘colonization resistance’ (Buffie and Pamer, 2013). Several mechanisms have been proposed to explain such symbiont-mediated interference with the growth of pathogens (Haine, 2008; Hamilton and Perlman, 2013). These involve exploitative competition between symbionts and pathogens for limiting factors such as nutrients (Maltby et al., 2013) and adhesion receptors (Juge, 2012). Furthermore, beneficial microbes can stimulate the host’s immune system against potential pathogens (Vaishnava et al., 2008), a mechanism analogous to apparent competition, in which an increase in one species causes an increase in a predator that negatively affects a competitor (Holt, 1977). Third, production of microbicidal factors is a common case of interference competition among bacteria. Numerous bacteriocins produced by the intestinal microbiota are active against potential pathogens such as Listeria, Salmonella and Clostridium species (Dabard et al., 2001; Gong et al., 2010; Rea et al., 2010). Cuticular Streptomycetes bacteria are protecting the offspring of digger wasps from fungal pathogens by producing a complex cocktail of antibiotics (Kroiss et al., 2010). The role of individual members of a highly diverse bacterial community associated with a host remains largely unclear. In addition, little is known about the effects that interplay between commensal bacteria might have. Better mechanistic insight into the interactions among the commensal microbiota in the epithelium is thus key.
Epithelia are the first line of defense against pathogenic microorganisms. As a barrier the epithelium has to coordinate physiological functions with the control of commensal microbes and the prevention of pathogenic infections. A characteristic feature of most animal epithelial cells is a dense carbohydrate-rich layer at the apical cell surface, referred to as the glycocalyx (Ouwerkerk et al., 2013). The glycocalyx represents a highly diverse and constantly renewed range of transmembrane glycoproteins, proteoglycans and glycolipids (Moran et al., 2011). As it excludes large molecules and organisms from direct access to the cell surface by steric hindrance, whereas smaller molecules might pass through, the glycocalyx represents the first line of contact between host cells and bacteria and viruses.
The cnidarian Hydra is a useful model to characterize a barrier epithelium, innate immune responses, tissue homeostasis and host–microbe interactions (Fraune and Bosch, 2007, 2010; Bosch et al., 2009; Fraune et al., 2009; Franzenburg et al., 2012; Bosch, 2013; Franzenburg, 2013b). Whereas polyps are colonized by a ‘low-complexity’ microbiota, the holobiont forms a highly specific ecosystem (Fraune and Bosch, 2007; Franzenburg et al., 2013b) that is similar between laboratory-raised animals and animals being taken from the wild (Fraune and Bosch, 2007). The ectodermal and endodermal epithelium is constantly renewed and the endodermal epithelium fulfills functions similar to that of the intestinal epithelium in mammals (Augustin and Bosch, 2010). The recognition of bacteria is mediated by an intermolecular interaction of HyLRR-2 as receptor and HyTRR-1 as signal transducer (Bosch et al., 2009). Upon activation, the receptor recruits the primary adaptor molecule MyD88 (myeloid differentiation factor 88). This receptor complex activation then triggers the innate immune response that involves the production of a variety of immune effector genes (Franzenburg et al., 2012). Antimicrobial peptides are major components of the innate immune system of Hydra (Augustin et al., 2009a; Bosch et al., 2009; Bosch, 2013). The expression of selective antimicrobial peptides is critical for colonization by stable and species-specific bacterial communities (Fraune et al., 2010; Franzenburg et al., 2013b). Intriguingly, most of the antimicrobial genes identified so far are expressed in the endodermal epithelium lining the gastric cavity (Augustin et al., 2009a, 2009b; Bosch et al., 2009). It remained to be shown, therefore, which mechanisms contribute to pathogen clearance in the ectodermal epithelium.
Here, we have used a gnotobiotic Hydra model to analyze the localization and importance of commensal bacteria in prevention of fungal infections. We identified commensal bacteria residing in the multilayered glycocalyx covering ectodermal epithelial cells. We also show that in the absence of these colonizers Hydra polyps are prone to fungal infection. Restoring the specific microbiota in gnotobiotic polyps prevents fungal infection. Strikingly, mono-associations with distinct members of the microbiota are not efficient or fail to provide protection. In contrast, synergistic and additive interactions of certain bacterial colonizers provide a significant resistance against Fusarium infections. Thus, bacteria–bacteria interactions within the commensal microbiota associated with the Hydra epithelium appear to be central to pathogen clearance.
Materials and methods
Animals
Experiments were carried out using Hydra vulgaris (AEP) (Hemmrich et al., 2007). All animals were cultured under constant temperature (18 °C), light conditions (12 h/12 h light/dark rhythm) and culture medium (0.28 mM CaCl2, 0.33 mM MgSO4, 0.5 mM NaHCO3 and 0.08 mM KCO3) according to the standard procedure (Lenhoff and Brown, 1970). The animals were fed three times a week with first instar larvae of A. salina. During recolonization and experimental infection with Fusarium sp., polyps were not fed.
Confocal microscopy of Hydra glycocalyx
Polyps were fixed in 2% paraformaldehyde, 2.5% glutaraldehyde and 75 mM L-lysine in 50 mM cacodylate buffer, pH 7.4, for 18 h at 4 °C. Animals were washed six times for 10 min in phosphate-buffered saline. After washing, polyps were stained with SYBR Gold (Life Technologies GmbH, Darmstadt, Germany) for 5 min. Before embedding in Mowiol/DABCO (Sigma-Aldrich, St Louis, MO, USA), animals were rinsed for 10 min in phosphate-buffered saline. Animals were analyzed using a Leica (Wetzlar, Germany) TCS SP5 confocal laser scanning microscope.
High-pressure freezing/freeze substitution fixation (HPF/FS) of Hydra glycocalyx
Hydra polyps immersed in Hydra culture medium were quickly dissected to fit into HPF specimen carriers. Tissue pieces are pipetted with Hydra culture medium into the cavity of a HPF aluminum platelet, which was 100 μm in depth and prefilled with 1-hexadecene. This platelet was covered by a second one, inserted into a HPF specimen holder and high-pressure frozen using HPM 010 (Bal-Tec, Balzers, Liechtenstein). Before FS, the frozen 1-hexadecene was carefully removed under liquid nitrogen, and then the samples were transferred into precooled test tubes filled with acetone containing 1% (w/v) OsO4 and 0.2% uranyl actetate. Dehydration was carried out at 90 °C in a conventional FS unit (AFS, Leica Microsystems, Vienna, Austria) for 24 h, followed by two further FS steps at 70 °C and 50 °C, each for 8 h. After FS, the temperature was raised up to 48 °C and samples were infiltrated at 48 °C with EPON according to the following protocol: specimens were (1) washed in pure acetone for 3 × 10 min, (2) infiltrated with 30% (v/v) EPON in acetone for 3 h, (3) then infiltrated with 70% EPON (v/v) in acetone for 3 h and (4) finally, three incubation steps in pure EPON, each for 2 h, were performed at room temperature. Ultrathin sections were counterstained with 2.5% uranyl acetate and lead citrate solution, and finally investigated in a Philips EM 208S transmission electron micrograph (Philips, Eindhoven, The Netherlands).
Chemical fixation of Hydra glycocalyx
Polyps were fixed in 2% paraformaldehyde, 2.5% glutaraldehyde, 75 mM L-lysine and 0.05% ruthenium red in 50 mM cacodylate buffer, pH 7.4, for 18 h at 4 °C. After washing with 75 mM cacodylate buffer for 30 min, postfixation was carried out with 1% OsO4 and 0.05% ruthenium red in 75 mM cacodylate buffer for 2 h at 4 °C. After washing with 75 mM cacodylate buffer for 30 min, tissue was dehydrated in ethanol. For scanning electron microscopy, animals were critical point dried in an ethanol–carbon dioxide mixture (CPD030; Bal-Tec), sputter coated (SCD050; Bal-Tec) and viewed at 10 kV using S420 scanning electron microscope (LEO, Leica).
For transmission electron microscopy, animals were embedded in Agar 100 resin (Agar Scientific, Ltd, Stansted, UK). Ultrathin sections were contrasted with 2.5% uranyl acetate for 5 min and lead citrate solution (freshly prepared from lead acetate and sodium citrate) for 2 min and were analyzed using a Tecnai G2 Spirit BioTWIN transmission electron microscope (FEI Company, Hillsboro, OR, USA).
Fluorescence in situ hybridization analysis of bacterial colonizers
Hydra polyps were washed in 500 μl phosphate-buffered saline for 2 min. The supernatant was transferred to a new tube and fixed by adding 500 μl 8% paraformaldehyde for 1 h. After fixation, supernatant was filtered through a white polycarbonate membrane filter (pore size: 0.2 μm). Afterwards, the filter was washed by 10 ml sterile H20 and air-dried. Hybridizations of filters were done as described by Manz et al. (1992) with monofluorescently labeled ribosomal RNA (rRNA)-targeted oligonucleotide probes: positive control, universal eubacterial probe EUB338 5′-GCTGCCTCCCGTAGGAGT-3′, and negative control, EUB338 antisense probe non-EUB338 5′-ACTCCTACGGGAGGCAGC-3′. The phylotype-specific oligonucleotide probes (Fraune et al., 2010) were designed using the computational tool Primrose 2.17 (Ashelford et al., 2002). Probes were 5′ end-labeled with either Alexa Fluor 488 (Life Technologies GmbH) (green fluorescence) or Cy3 (Life Technologies GmbH) (red fluorescence). Hybridization was carried out at 46 °C for 90 min followed by one wash step at 48 °C for 15 min. The formamide concentration in the hybridization buffer varied between 0% and 30%, and the sodium chloride concentration in the post-hybridization buffer was adjusted accordingly. The fluorescence signal by all probes was stable; the intensity of the signals was stable between 0% and 20% formamide and decreased slightly at 30% formamide. With nontarget cells, there was no signal even under low-stringency conditions (no formamide). Therefore, we routinely used 10% formamide for single hybridizations and for double hybridizations with EUB338. In addition, samples were stained with Hoechst staining and mounted with Citifluor (Citifluor Ltd, London, UK). Examination was done at magnification of × 600 with a Zeiss Axioskope 2 (Zeiss, Oberkochen, Germany).
Cultivation of Hydra-associated bacteria
Single Hydra polyps were placed in a 1.5-ml reaction tube and washed three times with 1 ml sterile filtered Hydra medium. After homogenization with a pestle, 100 μl (equates to 1/10 of a polyp) was plated on R2A agar plates (Sigma-Aldrich). After incubation at 18 °C for 5 days, single colony-forming units (CFUs) were isolated and cultivated in liquid R2A medium. The bacteria were identified by Sanger sequencing of the 16S rRNA gene and stocks were stored in Roti-Store cryo vials (Carl Roth, Karlsruhe, Germany) at −80 °C.
Isolation, culturing and identification of Fusarium sp.
Fungal hyphae were isolated from infected germ-free (GF) Hydra cultures and cultured on R2A agar plates at 18 °C for 3 days. Freshly grown hyphae were transferred to fresh agar plates or into a falcon tube containing 50 ml liquid R2A medium. For identification, fungal genomic DNA were extracted using polyps using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany). The internal transcribed spacer (ITS) of the ribosomal nuclear DNA was amplified using the universal ITS1 and ITS4 primer pair, as described in Paul and Steciow (2004). The fungi ITS was sequenced by Sanger sequencing and compared with public database at NCBI (National Center for Biotechnology Information) using blast searches. For phylogenetic analysis a sequence alignment for the ITS region was generated using MEGA5 (Tamura et al., 2007). A model test was used to estimate the best-fit substitution models for phylogenetic analyses. For the maximum-likelihood analyses, genes were tested using the Kimura 2-parameter model+G model. A bootstrap test with 100 replicates for maximum likelihood and random seed was conducted.
Plate diffusion assay to test the in vitro activity of isolated bacteria against Fusarium sp.
Six isolated bacteria were tested alone or in combinations in a plate diffusion assay for their in vitro activity against the isolated Fusarium fungi. Therefore, 10 μl of a pure bacterial culture (OD600=0.1) or a mixture of two bacterial culture (OD600=0.1) was spotted into small holes (3 mm) on R2A agar plates. After 2 days of bacterial growth, 10 μl of fungal spores (∼500 spores per μl) were added to the holes and fungal growth was quantified after 5 days by measuring the diameter of visible hyphae. Analyses of variance were used to test the effect of single bacterial isolates to fungal growth. Dunnett’s test was used for a post hoc test to compare treatment with control samples. Two-way analysis of variance was used to test the interaction effect (synergy or antagonism) of two bacterial isolates to fungal growth.
Generation of GF Hydra
Polyps were incubated for 1 week in an antibiotic solution containing 50 μg ml−1 each of ampicillin, rifampicin, streptomycin and neomycin with daily exchange of the solution. After 1 week of treatment, the polyps were transferred into sterile-filtered and autoclaved Hydra medium and fed with GF A. salina larvae (hatched in 30‰ artificial sea water containing the same antibiotic solution). Following 1 week of recovery, the absence of bacteria was verified by plating homogenized polyps on R2A agar plates. After incubation at 18 °C for 5 days, the CFUs were counted. Absence of CFUs indicated successful antibiotic treatment.
For culture-independent analysis, total DNA was extracted from single polyps using the DNeasy Blood & Tissue Kit (Qiagen). The 16S rRNA genes were amplified using the universal primers Eub-27F and Eub-1492R (Weisburg et al., 1991) in a 30-cycle PCR. Sterility was verified by the absence of a PCR product, whereas the positive control of none-treated polyps showed a signal.
Generation of mono- and di-associated Hydra
Bacteria isolated from Hydra polyps were cultured in liquid R2A medium for 3 days at 18 °C. Following centrifugation at 1380 × g for 10 min, the bacterial pellet was resuspended in sterile Hydra medium. Using a photometer, the optical density (OD600) of each bacterial solution was adjusted to 0.1. For di-associations, both bacterial solution were mixed in a 1:1 ratio. GF Hydra polyps were incubated in these solutions for 24 h. Conventionalized polyps were incubated in a mixture of Hydra vulgaris (AEP) culture supernatant and H. vulgaris (AEP) tissue homogenates (one homogenated polyp per ml) instead. Nonassociated bacteria were removed by washing with sterile Hydra medium after 24 h. Following another 24 h, the successful re-association was checked by plating tissue homogenates on R2A agar plates and counting CFU/polyps. Statistical analysis of the bacterial load was conducted using analysis of variance. Dunnett’s test was used as a post hoc test to compare treatment with control samples.
In vivo infection experiments with Fusarium sp.
The fungi Fusarium sp. was cultured on R2A agar plates. A piece of hyphae containing agar was transferred into a falcon tube, containing 50 ml liquid R2A medium. The tube was sealed and incubated at room temperature for 48 h. Fungal spores were retrieved from the supernatant and transferred into 1.5 ml reaction tubes. After centrifugation at 20 000 × g for 5 min, the pellet was resuspended in 1/10 of the original volume using sterile Hydra medium. For fungal infection, groups of five Hydra polyps were placed in a volume of 480 μl sterile Hydra medium using 1.5 ml tubes. Each treatment was repeated between 18 to 44 times (see Figure 5c). All re-associated Hydra polyps were infected with 20 μl spore solution (∼500 spores per μl) from the supernatant of a 48-h-old fungal culture. Fungal growth was monitored 7 days post infection by the outgrowth of hyphae. If fungal hyphae were detectable around the polyps, the tube was counted as ‘infected’. In case of no detectable hyphae, the tube was counted as ‘uninfected’. Statistical analyses were conducted by Fisher’s exact test to test whether bacterial recolonization of polyps caused different infection rates compared with GF or control polyps.
To test whether bacterial di-associations possess synergistic or antagonistic activities, we used a generalized linear model (function glm() from stats package in R), with individual infection as response. The different bacteria and all experimentally tested interactions were used as explanatory factors. We performed model selection using the drop1() function from the stats package. The best model was selected based on Akaike’s information criterion, a measure for the relative quality of a model. We used analysis of deviance for significance testing of the remaining factors within the chosen model (the model with the lowest Akaike’s information criterion). All significant interaction terms indicate synergistic or antagonistic effects of bacterial colonizers.
Results
The Hydra ectoderm is covered by a multilayered glycocalyx that is a habitat for a complex bacterial community
Using HPF/FS we first confirmed earlier observations (Holstein et al., 2010; Böttger et al., 2012) that the Hydra ectoderm is covered by a multilayered glycocalyx. Transmission electron microscopy revealed five distinct layers (c1–5) in the glycocalyx that together extend up to 1.5 μm from the cell surface (Figure 1b). The c1 layer is closely associated with the ectodermal cells, whereas the layer c5 is made by a loose meshwork accounting for >50% of the glycocalyx. Numerous electron-dense vesicles within the ectodermal epithelial cell (Figure 1b) indicate that glycocalyx components get secreted by ectodermal epithelial cells.
Hydra ectodermal glycocalyx is colonized by a complex bacterial community. (a) Schematic drawing of the freshwater polyp Hydra indicating the tissue areas in which the glycocalyx and the bacterial colonization was examined. The letters correspond to further panels in this figure. (b) Hydra ectodermal epithelial cells prepared by HPF/FS fixation provide excellent preservation of the glycocalyx layer revealing five distinct layers (c1–c5); pm, plasma membrane. (c) Total bacterial community colonizing the surface of the ectodermal epithelium in Hydra, stained with SYBR gold. (d, e) Raster electron micrograph (REM) of bacterial cells located on the surface of ectodermal cells. (f) Transmission electron micrograph (TEM) of a rod-shaped bacterium (red arrows) located within the outer layer (c5) of the glycocalyx covering ectodermal epithelial cells. (g–i) Fluorescence in situ hybridization (FISH) analysis of bacteria removed from the ectodermal epithelium. Bacteria cells were stained with the phylotype-specific probe for Curvibacter sp. (Curvi_442) (g) and with the eubacterial oligonucleotide probe EUB338 (h). Overlay images indicating the specifically labeled bacteria in yellow (i).
To localize the commensal microbiota, we initially used SYBR Gold staining. Epifluorescence microscopy uncovered (Figure 1c) a dense and morphologically heterogeneous community of bacteria colonizing the ectodermal epidermis. We next used scanning electron microscope investigations of chemical-fixed Hydra to better visualize the external appearance of the bacteria. In all specimens examined, rod-shaped as well as cocci bacteria were found attached to the ectodermal epithelial cells (Figures 1d and e), indicating that Hydra hosts a morphologically diverse microbial community. Next, we wanted to address whether bacterial colonizers live within the glycocalyx and if so where precisely. Transmission electron microscopy localized the commensal bacteria in the loose outer layer (c5) of the glycocalyx (Figure 1f), whereas the inner attached layers c1–4 were never observed to contain bacteria. This indicates that Hydra inner glycocalyx layers closely associated with the ectodermal epithelial cells are impenetrable to bacteria and may function as a protective barrier for the epithelial cell surface. The dominant member of the bacterial community colonizing H. vulgaris (AEP) tissue is Curvibacter spec. (Franzenburg et al., 2013b): Curvibacter sp. was co-sequenced with the Hydra magnipapillata genome (Chapman et al., 2010) and dominates 16S rRNA gene libraries in H. vulgaris (AEP) and H. magnipapillata (Franzenburg et al., 2013a, 2013b). To analyze whether Curvibacter is living within the glycocalyx, polyps were washed sequentially with high salts as previous observations in our laboratory indicated that the glycocalyx is shed under hypertonic conditions. The supernatant was subsequently fixed onto membrane surfaces and subjected to fluorescence in situ hybridization with phylotype-specific probes for Curvibacter sp. (Curvi442) (Fraune et al., 2010). This assay demonstrated (Figures 1g–i) that Curvibacter is a rod-shaped bacterium that is localized in the glycocalyx at the surface of the epithelium.
GF polyps are prone to infection by the filamentous fungus Fusarium sp.
Although the above observations suggest that Hydra surface is densely colonized by a distinct bacterial community, the role of the commensal bacteria that thrive on the ectodermal epithelium remained unclear. In order to address microbial functions in pathogen defense, we analyzed the outcome of fungal infection in GF Hydra. Although control H. vulgaris (AEP) cultures normally do not show any signs of fungal infection (Figure 2a), GF H. vulgaris (AEP) cultures are often infected by fungi (Figures 2b and c). Fungal hyphae are growing on the surface of GF polyps closely attached to the ectodermal epithelium-producing spores that subsequently can get released to the surrounding water (Figure 2c). In line with the hypothesis that members of the normal microbiota residing on the surface of the polyps may play a key role in pathogen defense, untreated fungal infections of GF polyps frequently cause the death of the animals.
GF Hydra polyps are prone to fungal infection by Fusarium sp. (a) Raster electron micrograph (REM) of a control polyp showing no fungal infection. (b) GF Hydra polyp infected by Fusarium sp. (c) Fungal hyphae in association with Hydra producing a spore. (d) Fungal hyphae grown in liquid R2A medium. (e) Spores isolated from the supernatant of a liquid Fusarium sp. culture. (f) Phylogenetic position of Fusarium sp. (isolated from infected Hydra) within the Nectriaceae (based on ITS region, maximum likelihood using Kimura 2-parameter model+G). Bootstrap values are shown at the corresponding nodes. The branch-length indicator displays 0.05 substitutions per site.
Using standard culturing conditions we isolated fungal hyphae from infected Hydra polyps to establish a pure fungal culture growing both on plates and in liquid medium (Figures 2d and e). Sequencing of the ITS (ITS1 and ITS2) ribosomal DNA identified the pathogenic fungi as Fusarium sp. (also known as Gibberella sp.) (Figure 2f), a filamentous fungus belonging to the order Hypocreales.
In vitro antifungal activity of single bacterial isolates
To dissect the pathogen defense potential of individual members of Hydra complex microbiota, we isolated and cultured six different bacterial strains from H. vulgaris (AEP) epithelium. In order to verify their host specificity, we analyzed their presence in 16S rRNA libraries (Franzenburg et al., 2013b). All six cultivated bacteria could be confirmed independently by a culture-independent method (454 pyrosequencing of 16S rRNA genes) to be present in the bacterial community of H. vulgaris (AEP) (Table 1). Whereas five bacterial strains belong to the Burkholderiales within the Betaproteobacteria, one bacterial strain belongs to the Pseudomonadales within the Gammaproteobacteria. These six cultivated bacteria represent 90.0±2.3% of the bacterial abundance in H. vulgaris (AEP) (Table 1) characterized previously (Franzenburg et al., 2013b). Therefore, these six cultivated bacteria are good representatives for the bacterial composition of H. vulgaris (AEP) that is dominated by Betaproteobacteria of the order Burkholderiales.
To monitor their impact on fungus growth, all six bacterial strains were investigated in an in vitro assay for the ability to prevent Fusarium sp. germination and mycelia growth. The activities of the isolated bacterial strains against the pathogenic fungus were examined by a dual-culture plate method. Interestingly, in this in vitro assay the majority of resident bacteria including the main colonizer Curvibacter showed only a minor or no ability to inhibit Fusarium sp. outgrowth after 5 days of incubation (Figure 3). Only one bacterial strain, Pelomonas sp., exhibit strong inhibitory activity in vitro against the pathogenic fungus.
In vitro activity of bacterial isolates against Fusarium sp. In vitro plate diffusion assay for fungal inhibition by bacteria isolated from Hydra tissue. Statistical analysis was conducted using analysis of variance (ANOVA; *P<0.05, **P<0.01, ***P>0.001; n=5. Acid., Acidovorax sp.; Curv., Curvibacter sp.; Duga., Duganella sp.; Pelo., Pelomonas sp.; Pseu., Pseudomonas sp.; Undi., Undibacterium sp.).
Bacteria–bacteria interactions increase antifungal activity in vitro
To assess the possibility that bacteria–bacteria interactions facilitate the observed antifungal resistance of control polyps, we tested pair-wise combinations of all six isolated bacteria in vitro. In comparing the antifungal activity of bacterial isolates alone with the activity of the pair-wise cultured bacteria, we were able to show that most of the co-cultures show a greater antifungal activity than the corresponding bacteria alone (Table 2). Two-way analysis of variance suggests that in most combinations the bacteria act in an additive manner against Fusarium sp. (Table 2 and Figure 4a). Interestingly, one bacterial combination (Undibacterium sp./Acidovorax sp.) acts synergistically to inhibit the fungal growth in vitro (Table 2 and Figure 4b). In contrast, two bacterial co-cultures (Pelomonas sp./Undibacterium sp. and Pelomonas sp./Duganella sp.) act in an antagonistic manner (Table 2 and Figure 4c). In both cases the strong antifungal activity of Pelomonas sp. alone is reduced in combination with each of the two other bacteria.
Examples of in vitro activity of co-cultured bacteria against Fusarium sp. (a) Example of an additive effect of two bacterial isolates in a plate diffusion assay. (b) Example of a synergistic effect of two bacterial isolates. (c) Example of an antagonistic effect of two bacterial isolates. Statistical analysis was conducted using two-way analysis of variance (ANOVA) to test the interaction effect (synergy or antagonism) of two bacterial isolates to fungal growth (*P<0.05, **P<0.01, ***P>0.001) (see also Table 2). Acid., Acidovorax sp.; Curv., Curvibacter sp.; Duga., Duganella sp.; Pelo., Pelomonas sp.; Pseu., Pseudomonas sp.; Undi., Undibacterium sp.
Bacteria–bacteria interactions are also needed in vivo to provide full protection
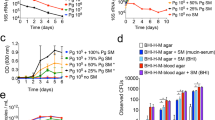
We wanted to address the in vivo relevance of the in vitro results and the importance of bacteria–bacteria interaction for the antifungal activity in the native Hydra host. To uncover this, we established a gnotobiotic Hydra model that was selectively colonized with one or two of the six bacterial strains (Figure 5a).
In vivo infection rates of Hydra polyps recolonized by different bacterial isolates. (a) Experimental set-up for mono- and di-associated and conventionalized (conv) Hydra polyps used for fungal infection experiments. (b) Bacterial load of recolonized Hydra polyps. N/A indicates ‘not available’ as Pseudomonas sp. shows swarming behavior and thereby overgrew Curvibacter sp., n≥4. (c) In vivo infection rates with Fusarium sp. after inoculation with spores. Statistical analyses were conducted by Fisher’s exact test. Different lowercase letters indicate significant differences between treatments: ‘a’ indicates significantly different from control (P<0.01), ‘b’ indicates significantly different from GF (P<0.01), ‘c’ indicates significantly different from control and GF (P<0.01). Fraction numbers indicate x infected cases per n replicates. Acid., Acidovorax sp.; Curv., Curvibacter sp.; Duga., Duganella sp.; Pelo., Pelomonas sp.; Pseu., Pseudomonas sp.; Undi., Undibacterium sp.
For di-associations, we always used Curvibacter sp. in combination with one of the five other bacterial isolates, as Curvibacter sp. is the most dominant colonizer (∼75%, see Table 1) in the natural bacterial community of H. vulgaris (AEP).
As controls we tested wild-type, conventionalized (that is, ex GF polyps re-infected with a complex microbiota) and GF polyps. To evaluate the effectivity of recolonization, we first monitored the bacterial load by assessing the bacterial CFUs per polyp (Figure 5b). In mono-association, only Acidovorax sp. showed increased bacterial load compared with control polyps. All other mono-associations resulted in bacterial loads comparable to control polyps, indicating that available niches can be colonized by all bacteria tested (Figure 5b). Similarly, only di-association with Curvibacter sp. and Acidovorax sp. yielded higher bacterial loads when compared with control polyps. All other di-associations showed no differences in bacterial load compared with control polyps (Figure 5b).
To examine antifungal activity in Hydra that were selectively colonized with one or two of the six bacterial strains, polyps were screened for the presence or absence of fungal hyphae 7 days post infection with 20 μl spore solution (∼500 spores per μl). To test for differences in infection rates between controls (GF and control) and recolonized polyps, we used Fisher’s exact test. We compared all treatments with GF and control polyps, respectively (Figure 5c). As shown in Figure 5d, GF polyps were highly susceptible to fungal outgrowth, whereas control polyps largely inhibited fungal growth. The re-introduced complex microbiota (conventionalized) provided the same resistance against fungal infection as observed in control polyps, indicating that the resident microbiota facilitates fungal clearance and also that the antibiotic treatment per se does not lead to host tissue damage to foster Fusarium outgrowth (Figure 5c).
Individual effects of single bacterial isolates were significant for Curvibacter sp., Undibacterium sp., Acidovorax sp. and Pelomonas sp compared with GF polyps (Figure 5c). Strikingly, none of the mono-associated polyps provided the polyps with the same rate of resistance as control or conventionalized polyps. Surprisingly, Pelomonas sp., which inhibits fungal growth significantly in vitro (Figure 3), showed no antifungal activity in vivo (Figure 5c). Vice versa, Acidovorax sp., which showed only weak effect in vitro, appears to have strong antifungal activity in vivo.
Within the di-associations, three combinations possess a significant activity against fungal infection compared with GF polyps (Figure 5c). Interestingly, two combinations (Curvibacter sp./Duganella sp. and Curvibacter sp./Pelomonas sp.) are as active as control polyps against fungal infections. In contrast, two combinations (Curvibacter sp./Acidovorax sp. and Curvibacter sp./Pseudomonas sp.) exhibit no antifungal activity (Figure 5c).
To evaluate the contribution of bacteria–bacteria interaction to the observed antifungal activity in di-associations in more detail, we used a generalized linear model (Table 3). We found three significant bacteria–bacteria interactions contributing to fungal infection rates in di-associations (Table 3). Whereas two interactions, Curvibacter sp./Undibacterium sp. and Curvibacter sp./Acidovorax sp., exhibit an antagonistic effect on fungal infection, the combination of Curvibacter sp./Duganella sp. exhibit a synergistic effect (Table 3). The strong reduction of infection rate of the combination of Curvibacter sp./Pelomoans sp. can be explained by an additive effect of the individual effects of both bacteria.
Discussion
In this study, we have examined the localization and pathogenic fungus clearance potential of members of H. vulgaris (AEP) resident microbiota. We found that the bacterial colonizers in Hydra inhabit the outer layer of the glycocalyx and, therefore, appear to have no direct contact to the ectodermal epithelium (Figure 1f). Thus, the glycocalyx seems on one hand to separate the bacterial cells from the epithelium and on the other hand to provide a habitat for the bacterial colonizers. This principle of separation into a habitat for symbiotic bacteria and a physical barrier preventing excessive immune activation was previously described for the mucosal surface of the mammalian colon, where a mucous layer is restricting bacterial colonizers to the outer loose mucus layer whereas the inner mucus layer is devoid of bacteria (Johansson et al., 2008, 2011). As such a glycoprotein-covered barrier epithelium can be traced back to the ancestral metazoan Hydra, it apparently is a conserved feature shared by many multicellular animals.
We also discovered that Hydra polyps, when artificially deprived of their specific bacterial colonizers, are prone to fungal infection by the filamentous fungus Fusarium sp. Spores of Fusarium sp. seem to be continuously present in the laboratory environment surrounding the Hydra polyp. Our observations indicate that the specific microbiota (Fraune and Bosch, 2007; Franzenburg et al., 2013b) colonizing the interface between Hydra host ectodermal epithelium and the environment provide efficient protection against fungal infection.
We identified several bacterial colonizers, including Acidovorax sp., Curvibacter sp., Pelomonas sp. and Undibacterium sp., that significantly inhibit fungal outgrowth in vivo (Figure 5c). None of these bacteria were previously reported to synthesize antifungal compounds, although Acidovorax sp. and Curvibacter sp. are reported as symbionts in other organisms (Schramm et al., 2003; McKenzie et al., 2012). Most importantly, we have observed that none of the tested bacterial colonizers alone was able to provide full antifungal resistance (Figure 5c). In contrast, resistance, observed in control polyps, was achieved in polyps recolonized by a complex bacterial community (conventionalized) indicating that bacteria–bacteria interactions contribute to the full resistance against fungal infections. Our in vivo and in vitro results indicate that bacterial colonizers of H. vulgaris (AEP) interact in a complex manner and that the sum of additive, synergistic as well as antagonistic effects may gives rise to the overall resistance of the holobiont Hydra against fungal infections. Interestingly, the two most dominant bacterial colonizers Curvibacter sp. and Duganella sp. exhibit weak or no activity alone, but exhibit a strong synergistic effect in di-association, reducing the rate of infected polyps to 15%. This fact points to the in vivo importance of these two main colonizers for fungal clearance. In sum, this study provides first experimental evidence for the view that in animals at the base of metazoan evolution a complex microbiota is necessary and sufficient for pathogen clearance. The study also demonstrates that mono-associated bacteria in most cases fail to function efficiently in pathogen defense.
The observations in Hydra are in line with studies in the locust Schistocerca gregaria where species-rich bacterial communities provide better protection against pathogen invasion than species-poor communities (Dillon et al., 2005). The findings make it likely that an ‘unfavorable’ microbiota composition or fluctuating bacterial community composition may result in disturbed immune function of the whole metaorganism. To ensure continuous protection by specific bacteria, host mechanisms controlling bacterial colonization are required. In Hydra, we have shown that the expression of species-specific antimicrobial peptides are key factors in maintaining a species-specific bacterial colonization (Fraune et al., 2010; Franzenburg et al., 2013b). In addition, active immune signaling via the Toll-like receptor cascade is involved in the re-establishment of bacterial homeostasis following disturbance (Franzenburg et al., 2012) and, therefore, enhances the resilience of the bacterial community in Hydra.
Interestingly, the in vivo antifungal activity did not match the results obtained from in vitro experiments. To explain this discrepancy we offer four possible scenarios. First, certain Hydra-associated bacteria induce the production of host-derived antifungal compounds in Hydra. Second, the bacterial population density and the ratio of both bacteria in co-culture may differ between in vitro and in vivo experiments. As it was not possible to estimate the ratio of bacteria in co-culture as most tested bacteria morphologically do not differ significantly on agar plates, quantitative real-time PCR assays for unequivocal identification of the bacteria are under investigation. Third, the bacterial symbionts produce the antifungal compound only in association with the Hydra tissue, likely altering their metabolic state when changing their lifestyle from a free-living state to an epithelium colonizer. Fourth, antifungal compounds produced by the host and by the bacterial symbionts act together to inhibit fungal growth (Myers et al., 2012). Collectively, the observed differences between the in vitro and in vivo data suggest that simplified measures of in vitro microbial function may be insufficient or even misleading for evaluating the pathogen clearance potential of resident microbes.
How does the microbiota efficiently prevent growth of pathogenic fungi? The contributions of specific bacteria-derived molecules to immune defense against fungal pathogens are just beginning to be deciphered. Observations in a number of animal models provide hints that many associated symbionts serve a direct protective function for their host against fungal infections by producing antifungal substances. For example, embryos of the crustacean species Palaemon macrodactylus are colonized by symbiotic bacteria producing a secondary metabolite that is active against a pathogenic fungus (Gil-Turnes et al., 1989). A different example is the infectious disease chytridiomycosis, caused by the fungal pathogen Batrchochytrium dendrobatis, that is a major factor responsible for the worldwide decline of amphibian species (Skerratt et al., 2007). In this well-studied case, commensal bacteria have been shown to inhibit the growth of B. dendrobatis by the production of antifungal molecules like indole-3-carboxaldehyde or violacein (Brucker et al., 2008; Harris et al., 2009). Susceptibility to B. dendrobatis infection varies among amphibian species, and even within species some populations can coexist with B. dendrobatis whereas others decline to extinction. These differences in disease susceptibility have been correlated with the diversity of antifungal bacteria associated with a given frog population (Woodhams et al., 2007). Interestingly, Curvibacter species are also associated with a variety of amphibian species (McKenzie et al., 2012; Loudon et al., 2013), but were not yet shown to produce antifungal compounds. Another prominent example for fungal defense by symbiotic bacteria is present in fungus-growing ants. These ants grow fungal cultivars for their nutrition that are prone to infection by the parasitic fungus Escovopsis sp. To defend their fungal cultivar against Escovopsis sp., leaf-cutter ants use symbiotic actinobacteria of the genus Pseudonochardia that are housed in specialized cuticular structure on the ant’s body (Caldera et al., 2009). These symbiotic bacteria produce the cyclic depsipeptide dentigerumycin that acts highly specific against Escovopsis sp., without harming the fungal cultivar (Oh et al., 2009). Thus, symbiotic bacteria are an integral part of antifungal immunity in a variety of organisms, offering an opportunity to resist fungal infection by a spread of bacterial symbionts.
The observations also support the view that because of bacterial colonizers Hydra might be able to adapt to new environmental conditions much faster than by genomic recombination. Thus, the microbiota is a complex trait that is under strong host genetic control. The resilience of complex and specific bacterial communities may be a critical factor to host health.
References
Ashelford KE, Weightman AJ, Fry JC . (2002). PRIMROSE: a computer program for generating and estimating the phylogenetic range of 16S rRNA oligonucleotide probes and primers in conjunction with the RDP-II database. Nucleic Acids Res 30: 3481–3489.
Augustin R, Anton-Erxleben F, Jungnickel S, Hemmrich G, Spudy B, Podschun R et al. (2009). Activity of the novel peptide arminin against multiresistant human pathogens shows the considerable potential of phylogenetically ancient organisms as drug sources. Antimicrob Agents Chemother 53: 5245–5250.
Augustin R, Bosch TCG . (2010). Cnidarian immunity: a tale of two barriers. Adv Exp Med Biol 708: 1–16.
Augustin R, Siebert S, Bosch TCG . (2009). Identification of a kazal-type serine protease inhibitor with potent anti-staphylococcal activity as part of Hydra’s innate immune system. Dev Comp Immunol 33: 830–837.
Bohnhoff M, Drake BL, Miller CP . (1955). The effect of an antibiotic on the susceptibility of the mouse’s intestinal tract to Salmonella infection. Antibiot Annu 3: 453–455.
Bosch TC, Augustin R, Anton-Erxleben F, Fraune S, Hemmrich G, Zill H et al. (2009). Uncovering the evolutionary history of innate immunity: the simple metazoan Hydra uses epithelial cells for host defence. Dev Comp Immunol 33: 559–569.
Bosch TCG . (2013). Cnidarian-microbe interactions and the origin of innate immunity in metazoans. Annu Rev Microbiol 67: 499–518.
Böttger A, Doxey AC, Hess MW, Pfaller K, Salvenmoser W, Deutzmann R et al. (2012). Horizontal gene transfer contributed to the evolution of extracellular surface structures: the freshwater polyp Hydra is covered by a complex fibrous cuticle containing glycosaminoglycans and proteins of the PPOD and SWT (sweet tooth) families. PLoS One 7: e52278.
Brucker RM, Harris RN, Schwantes CR, Gallaher TN, Flaherty DC, Lam BA et al. (2008). Amphibian chemical defense: antifungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus. J Chem Ecol 34: 1422–1429.
Buffie CG, Pamer EG . (2013). Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 13: 790–801.
Caldera EJ, Poulsen M, Suen G, Currie CR . (2009). Insect symbioses: a case study of past, present, and future fungus-growing ant research. Environ Entomol 38: 78–92.
Chapman JA, Kirkness EF, Simakov O, Hampson SE, Mitros T, Weinmaier T et al. (2010). The dynamic genome of Hydra. Nature 464: 592–596.
Currie CR, Poulsen M, Mendenhall J, Boomsma JJ, Billen J . (2006). Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science 311: 81–83.
Dabard J, Bridonneau C, Phillipe C, Anglade P, Molle D, Nardi M et al. (2001). Ruminococcin A, a new lantibiotic produced by a Ruminococcus gnavus strain isolated from human feces. Appl Environ Microbiol 67: 4111–4118.
Dillon RJ, Vennard CT, Buckling A, Charnley AK . (2005). Diversity of locust gut bacteria protects against pathogen invasion. Ecol Lett 8: 1291–1298.
Dobber R, Hertogh-Huijbregts A, Rozing J, Bottomly K, Nagelkerken L . (1992). The involvement of the intestinal microflora in the expansion of CD4+ T cells with a naive phenotype in the periphery. Dev Immunol 2: 141–150.
Douglas AE, Minto LB, Wilkinson TL . (2001). Quantifying nutrient production by the microbial symbionts in an aphid. J Exp Biol 204: 349–358.
Douglas AE, Wilkinson TL . (1998). Host cell allometry and regulation of the symbiosis between pea aphids, Acyrthosiphon pisum, and bacteria, Buchnera. J Insect Physiol 44: 629–635.
Franzenburg S, Fraune S, Altrock PM, Künzel S, Baines JF, Traulsen A et al. (2013). Bacterial colonization of Hydra hatchlings follows a robust temporal pattern. ISME J 7: 781–790.
Franzenburg S, Fraune S, Künzel S, Baines JF, Domazet-Loso T, Bosch TCG . (2012). MyD88-deficient Hydra reveal an ancient function of TLR signaling in sensing bacterial colonizers. Proc Natl Acad Sci USA 109: 19374–19379.
Franzenburg S, Walter J, Künzel S, Wang J, Baines JF, Bosch TCG et al. (2013). Distinct antimicrobial peptide expression determines host species-specific bacterial associations. Proc Natl Acad Sci USA 110: E3730–E3738.
Fraune S, Abe Y, Bosch TCG . (2009). Disturbing epithelial homeostasis in the metazoan Hydra leads to drastic changes in associated microbiota. Environ Microbiol 11: 2361–2369.
Fraune S, Augustin R, Anton-Erxleben F, Wittlieb J, Gelhaus C, Klimovich VB et al. (2010). In an early branching metazoan, bacterial colonization of the embryo is controlled by maternal antimicrobial peptides. Proc Natl Acad Sci USA 107: 18067–18072.
Fraune S, Bosch TCG . (2007). Long-term maintenance of species-specific bacterial microbiota in the basal metazoan Hydra. Proc Natl Acad Sci USA 104: 13146–13151.
Fraune S, Bosch TCG . (2010). Why bacteria matter in animal development and evolution. BioEssays 32: 571–580.
Gil-Turnes MS, Hay ME, Fenical W . (1989). Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science 246: 116–118.
Gong H-S, Meng X-C, Wang H . (2010). Mode of action of plantaricin MG, a bacteriocin active against Salmonella typhimurium. J Basic Microbiol 50 (Suppl 1): S37–S45.
Haine ER . (2008). Symbiont-mediated protection. Proc Biol Sci 275: 353–361.
Hamilton PT, Perlman SJ . (2013). Host defense via symbiosis in Drosophila. PLoS Pathog. 9: e1003808.
Harris RN, Brucker RM, Walke JB, Becker MH, Schwantes CR, Flaherty DC et al. (2009). Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J 3: 818–824.
Hemmrich G, Anokhin B, Zacharias H, Bosch TC . (2007). Molecular phylogenetics in Hydra, a classical model in evolutionary developmental biology. Mol Phylogenet Evol 44: 281–290.
Holstein TW, Hess MW, Salvenmoser W . (2010). Preparation techniques for transmission electron microscopy of Hydra. Methods Cell Biol 96: 285–306.
Holt RD . (1977). Predation, apparent competition, and the structure of prey communities. Theor Popul Biol 12: 197–29.
Human Microbiome Project Consortium. (2012). Structure, function and diversity of the healthy human microbiome. Nature 486: 207–214.
Johansson MEV, Ambort D, Pelaseyed T, Schütte A, Gustafsson JK, Ermund A et al. (2011). Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci 68: 3635–3641.
Johansson MEV, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC . (2008). The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA 105: 15064–15069.
Jones RM, Luo L, Ardita CS, Richardson AN, Kwon YM, Mercante JW et al. (2013). Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J 32: 3017–3028.
Juge N . (2012). Microbial adhesins to gastrointestinal mucus. Trends Microbiol 20: 30–39.
Kroiss J, Kaltenpoth M, Schneider B, Schwinger M-G, Hertweck C, Maddula RK et al. (2010). Symbiotic Streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat Chem Biol 6: 261–263.
Lenhoff HM, Brown RD . (1970). Mass culture of hydra: an improved method and its application to other aquatic invertebrates. Lab Anim 4: 139–154.
Loudon AH, Woodhams DC, Parfrey LW, Archer H, Knight R, McKenzie V et al. (2013). Microbial community dynamics and effect of environmental microbial reservoirs on red-backed salamanders (Plethodon cinereus). ISME J 8: 830–840.
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R . (2012). Diversity, stability and resilience of the human gut microbiota. Nature 489: 220–230.
Maltby R, Leatham-Jensen MP, Gibson T, Cohen PS, Conway T . (2013). Nutritional basis for colonization resistance by human commensal Escherichia coli strains HS and Nissle 1917 against E. coli O157:H7 in the mouse intestine. PLoS One 8: e53957.
Manz W, Amann R, Ludwig W, Wagner M, Schleifer KH . (1992). Phylogenetic oligdeoxynucleotide probes for the major subclasses for proteobacteria—problems and solutions. Syst Appl Microbiol 15: 593–600.
Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL . (2005). An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122: 107–118.
McFall-Ngai M, Hadfield MG, Bosch TCG, Carey HV, Domazet-Lošo T, Douglas AE et al. (2013). Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci USA 110: 3229–3236.
McKenzie VJ, Bowers RM, Fierer N, Knight R, Lauber CL . (2012). Co-habiting amphibian species harbor unique skin bacterial communities in wild populations. ISME J 6: 588–596.
Moran AP, Gupta A, Joshi L . (2011). Sweet-talk: role of host glycosylation in bacterial pathogenesis of the gastrointestinal tract. Gut 60: 1412–1425.
Myers JM, Ramsey JP, Blackman AL, Nichols AE, Minbiole KPC, Harris RN . (2012). Synergistic inhibition of the lethal fungal pathogen Batrachochytrium dendrobatidis: the combined effect of symbiotic bacterial metabolites and antimicrobial peptides of the frog Rana muscosa. J Chem Ecol 38: 958–965.
Oh D-C, Poulsen M, Currie CR, Clardy J . (2009). Dentigerumycin: a bacterial mediator of an ant-fungus symbiosis. Nat Chem Biol 5: 391–393.
Ouwerkerk JP, de Vos WM, Belzer C . (2013). Glycobiome: bacteria and mucus at the epithelial interface. Best Pract Res Clin Gastroenterol 27: 25–38.
Paul B, Steciow MM . (2004). Saprolegnia multispora, a new oomycete isolated from water samples taken in a river in the Burgundian region of France. FEMS Microbiol Lett 237: 393–398.
Rawls JF, Samuel BS, Gordon JI . (2004). Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci USA 101: 4596–4601.
Rea MC, Sit CS, Clayton E, O’Connor PM, Whittal RM, Zheng J et al. (2010). Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc Natl Acad Sci USA 107: 9352–9357.
Sandström J, Telang A, Moran NA . (2000). Nutritional enhancement of host plants by aphids—a comparison of three aphid species on grasses. J Insect Physiol 46: 33–40.
Schramm A, Davidson SK, Dodsworth JA, Drake HL, Stahl DA, Dubilier N . (2003). Acidovorax-like symbionts in the nephridia of earthworms. Environ Microbiol 5: 804–809.
Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD et al. (2007). Spread of Chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth 4: 125–134.
Sommer F, Bäckhed F . (2013). The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 11: 227–238.
Tamura K, Dudley J, Nei M, Kumar S . (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599.
Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV . (2008). Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA 105: 20858–20863.
Weisburg WG, Barns SM, Pelletier DA, Lane DJ . (1991). 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173: 697–703.
Weiss BL, Maltz M, Aksoy S . (2012). Obligate symbionts activate immune system development in the tsetse fly. J Immunol 188: 3395–3403.
Woodhams DC, Vredenburg VT, Simon M-A, Billheimer D, Shakhtour B, Shyr Y et al. (2007). Symbiotic bacteria contribute to innate immune defenses of the threatened mountain yellow-legged frog, Rana muscosa. Biol Conserv 138: 390–398.
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M et al. (2012). Human gut microbiome viewed across age and geography. Nature 486: 222–227.
Acknowledgements
We thank Antje Thomas for excellent technical assistance in electron microscopy, and Philipp Dirksen and Jan von Rönn for their assistance in glm modeling. This study was supported by Grants FR 3041/2-1 and Bo 848/17-1 from the Deutsche Forschungsgemeinschaft (DFG) and several grants from the DFG Excellence initiative (to TCGB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fraune, S., Anton-Erxleben, F., Augustin, R. et al. Bacteria–bacteria interactions within the microbiota of the ancestral metazoan Hydra contribute to fungal resistance. ISME J 9, 1543–1556 (2015). https://doi.org/10.1038/ismej.2014.239
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2014.239
This article is cited by
-
The reef-building coral Galaxea fascicularis: a new model system for coral symbiosis research
Coral Reefs (2023)
-
Symbiont transmission in marine sponges: reproduction, development, and metamorphosis
BMC Biology (2022)
-
Microbiota mediated plasticity promotes thermal adaptation in the sea anemone Nematostella vectensis
Nature Communications (2022)
-
Cultivation of gut microorganisms of the marine ascidian Halocynthia roretzi reveals their potential roles in the environmental adaptation of their host
Marine Life Science & Technology (2022)
-
Beyond Lynn Margulis’ green hydra
Symbiosis (2022)