Abstract
Background and objective:
CD36 is implicated in fatty-acid uptake in multiple tissues, including hepatocytes and adipocytes. Circulating CD36 (sCD36) is increased in non-alcoholic fatty liver disease (NAFLD). We explored this association further by investigating correlations between sCD36 levels, intrahepatic lipid content and markers of obesity in NAFLD patients and controls.
Methods:
In total, 111 NAFLD patients and 33 normal/overweight controls were included. Intrahepatic lipid content was measured by magnetic resonance spectroscopy; and subgroups of participants had a dual-energy X-ray absorptiometry (n=99), magnetic resonance imaging (n=94, subcutaneous and visceral adipose tissue) and liver biopsy (n=28 NAFLD patients) performed. Plasma sCD36 was assessed by enzyme-linked immunosorbent assay.
Results:
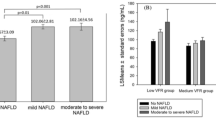
NAFLD patients had elevated sCD36 levels compared with controls (0.68 (0.12–2.27) versus 0.43 (0.10–1.18), P<0.01). sCD36 correlated with intrahepatic lipid (rs=0.30), alanine transaminase (ALT) (r=0.31), homeostasis model assessment index-insulin resistance (r=0.24), high-density lipoprotein (r=−0.32) and triglyceride (r=0.44, all P<0.01). Intrahepatic lipid and plasma triglyceride were independent predictors of sCD36 levels in a multiple regression analysis. Further, sCD36 and body mass index were weakly correlated (r=0.17, P=0.04); yet, we found no correlations between sCD36 and other measures of fat distribution except an inverse relation to visceral adipose tissue (rs=−0.21, P<0.05). We observed a trend for correlation between sCD36 and hepatic CD36 mRNA expression (r=0.37, P=0.07).
Conclusions:
sCD36 levels increased with the level of intrahepatic lipid, insulin resistance and dyslipidemia. The weak association with markers of obesity and the association with hepatic CD36 mRNA expression suggest that excess sCD36 in NAFLD patients is derived from the hepatocytes, which may support that CD36 is involved in NAFLD development. An unhealthy and unbalanced CD36 expression in adipose and hepatic tissue may shift the fatty-acid load to the liver.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 766–781.
Mottin CC, Moretto M, Padoin AV, Swarowsky AM, Toneto MG, Glock L et al. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg 2004; 14: 635–637.
Welsh JA, Karpen S, Vos MB . Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr 2013; 162: 496–500 e1.
Vernon G, Baranova A, Younossi ZM . Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34: 274–285.
Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ . Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005; 115: 134351.
Koonen DP, Jensen MK, Handberg A . Soluble CD36- a marker of the (pathophysiological) role of CD36 in the metabolic syndrome? Arch Physiol Biochem 2011; 117: 57–63.
Miquilena-Colina ME, Lima-Cabello E, Sanchez-Campos S, Garcia-Mediavilla MV, Fernandez-Bermejo M, Lozano-Rodriguez T et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 2011; 60: 1394–1402.
Garcia-Monzon C, Lo Iacono O, Crespo J, Romero-Gomez M, Garcia-Samaniego J, Fernandez-Bermejo M et al. Increased soluble CD36 is linked to advanced steatosis in nonalcoholic fatty liver disease. Eur J Clin Invest 2014; 44: 65–73.
Handberg A, Højlund K, Gastaldelli A, Flyvbjerg A, Dekker JM, Petrie J et al. Plasma sCD36 is associated with markers of atherosclerosis, insulin resistance and fatty liver in a nondiabetic healthy population. J Intern Med 2012; 271: 294–304.
Heebøll S, Kreuzfeldt M, Hamilton-Dutoit S, Kjær Poulsen M, Stødkilde-Jørgensen H, Møller HJ et al. Placebo-controlled, randomised clinical trial: high-dose resveratrol treatment for non-alcoholic fatty liver disease. Scand J Gastroenterol 2016; 51: 456–464.
Ornstrup MJ, Harsløf T, Kjær TN, Langdahl BL, Pedersen SB . Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: a randomized placebo-controlled trial. J Clin Endocrinol Metab 2014; 99: 4720–4729.
Poulsen MK, Nellemann B, Stødkilde-Jørgensen H, Pedersen SB, Grønbæk H, Nielsen S . Impaired insulin suppression of VLDL-triglyceride kinetics in non-alcoholic fatty liver disease. J Clin Endocrinol Metab 2016; 101: 1637–1646.
Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 2005; 288: E462–E468.
Ornstrup MJ, Kjær TN, Harsløf T, Stødkilde-Jørgensen H, Hougaard DM, Cohen A et al. Adipose tissue, estradiol levels, and bone health in obese men with metabolic syndrome. Eur J Endocrinol 2015; 172: 205–216.
Wallace TM, Levy JC, Matthews DR . Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487–1495.
Handberg A, Levin K, Hojlund K, Beck-Nielsen H . Identification of the oxidized low-density lipoprotein scavenger receptor CD36 in plasma: a novel marker of insulin resistance. Circulation 2006; 114: 1169–1176.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313–1321.
Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 2012; 56: 1751–1759.
Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA 2009; 106: 15430–15435.
Greco D, Kotronen A, Westerbacka J, Puig O, Arkkila P, Kiviluoto T et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol 2008; 294: G1281–G1287.
Bechmann LP, Gieseler RK, Sowa JP, Kahraman A, Erhard J, Wedemeyer I et al. Apoptosis is associated with CD36/fatty acid translocase upregulation in non-alcoholic steatohepatitis. Liver Int 2010; 30: 850–859.
Sheedfar F, Sung MM, Aparicio-Vergara M, Kloosterhuis NJ, Miquilena-Colina ME, Vargas-Castrillon J et al. Increased hepatic CD36 expression with age is associated with enhanced susceptibility to nonalcoholic fatty liver disease. Aging 2014; 6: 281–295.
Krssak M, Hofer H, Wrba F, Meyerspeer M, Brehm A, Lohninger A et al. Non-invasive assessment of hepatic fat accumulation in chronic hepatitis C by 1H magnetic resonance spectroscopy. Eur J Radiol 2010; 74: e60–e66.
Knøsgaard L, Thomsen SB, Stockel M, Vestergaard H, Handberg A . Circulating sCD36 is associated with unhealthy fat distribution and elevated circulating triglycerides in morbidly obese individuals. Nutr Diabetes 2014; 4: e114.
Liani R, Halvorsen B, Sestili S, Handberg A, Santilli F, Vazzana N et al. Plasma levels of soluble CD36, platelet activation, inflammation, and oxidative stress are increased in type 2 diabetic patients. Free Radic Biol Med 2012; 52: 1318–1324.
Lykkeboe S, Larsen AL, Handberg A . Lack of consistency between two commercial ELISAs and against an in-house ELISA for the detection of CD36 in human plasma. Clin Chem Lab Med 2012; 50: 1071–1074.
Bieghs V, Verheyen F, van Gorp PJ, Hendrikx T, Wouters K, Lutjohann D et al. Internalization of modified lipids by CD36 and SR-A leads to hepatic inflammation and lysosomal cholesterol storage in Kupffer cells. PloS One 2012; 7: e34378.
Bieghs V, Wouters K, van Gorp PJ, Gijbels MJ, de Winther MP, Binder CJ et al. Role of scavenger receptor A and CD36 in diet-induced nonalcoholic steatohepatitis in hyperlipidemic mice. Gastroenterology 2010; 138: 2477–2486 e1−3.
Glatz JF, Luiken JJ, Bonen A . Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev 2010; 90: 367–417.
Liang CP, Han S, Okamoto H, Carnemolla R, Tabas I, Accili D et al. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest 2004; 113: 764–773.
Steneberg P, Sykaras AG, Backlund F, Straseviciene J, Soderstrom I, Edlund H . Hyperinsulinemia enhances hepatic expression of the fatty acid transporter Cd36 and provokes hepatosteatosis and hepatic insulin resistance. J Biol Chem 2015; 290: 19034–19043.
Buque X, Cano A, Miquilena-Colina ME, Garcia-Monzon C, Ochoa B, Aspichueta P . High insulin levels are required for FAT/CD36 plasma membrane translocation and enhanced fatty acid uptake in obese Zucker rat hepatocytes. Am J Physiol Endocrinol Metab 2012; 303: E504–E514.
Nicholson AC . Expression of CD36 in macrophages and atherosclerosis: the role of lipid regulation of PPARgamma signaling. Trends Cardiovasc Med 2004; 14: 8–12.
Glintborg D, Højlund K, Andersen M, Henriksen JE, Beck-Nielsen H, Handberg A . Soluble CD36 and risk markers of insulin resistance and atherosclerosis are elevated in polycystic ovary syndrome and significantly reduced during pioglitazone treatment. Diabetes Care 2008; 31: 328–334.
Handberg A, Norberg M, Stenlund H, Hallmans G, Attermann J, Eriksson JW . Soluble CD36 (sCD36) clusters with markers of insulin resistance, and high sCD36 is associated with increased type 2 diabetes risk. J Clin Endocrinol Metab 2010; 95: 1939–1946.
Alkhatatbeh MJ, Mhaidat NM, Enjeti AK, Lincz LF, Thorne RF . The putative diabetic plasma marker, soluble CD36, is non-cleaved, non-soluble and entirely associated with microparticles. J Thromb Haemost 2011; 9: 844–851.
Gustafson CM, Shepherd AJ, Miller VM, Jayachandran M . Age- and sex-specific differences in blood-borne microvesicles from apparently healthy humans. Biol Sex Differ 2015; 6: 10.
Alkhatatbeh MJ, Enjeti AK, Acharya S, Thorne RF, Lincz LF . The origin of circulating CD36 in type 2 diabetes. Nutr Diabetes 2013; 3: e59.
Zhou D, Samovski D, Okunade AL, Stahl PD, Abumrad NA, Su X . CD36 level and trafficking are determinants of lipolysis in adipocytes. FASEB J 2012; 26: 4733–4742.
Acknowledgements
We are thankful for the skilled technical assistance provided by Lone Larsen, Aarhus University Hospital, Denmark. The study was supported by the NOVO Nordisk Foundation, Aarhus University, the Danish Council for Independent Research, Medical Sciences (11-107912); 'Savværksejer Jeppe Juhl og hustru Ovita Juhls mindelegat'. The study is part of the research program LIRMOI Research Center (www.LIRMOI.com), which is supported by the Danish Council for Strategic Research (0-093499).
Author contributions
Declaration of contribution: SH, HG and AH conceived the study. SH collected and researched data and wrote the manuscript. MKP, MO and TNK collected the data and reviewed/edited the manuscript. SN, SBP and HG assisted in study design, data interpretation and reviewed/edited the manuscript. AH assisted in collecting and researching data and writing the manuscript. AH and HG shares last co-author ship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Heebøll, S., Poulsen, M., Ornstrup, M. et al. Circulating sCD36 levels in patients with non-alcoholic fatty liver disease and controls. Int J Obes 41, 262–267 (2017). https://doi.org/10.1038/ijo.2016.223
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.223
This article is cited by
-
Understanding lipotoxicity in NAFLD pathogenesis: is CD36 a key driver?
Cell Death & Disease (2020)
-
Bariatric surgery reduces CD36-bearing microvesicles of endothelial and monocyte origin
Nutrition & Metabolism (2018)
-
Anthropometric and blood parameters for the prediction of NAFLD among overweight and obese adults
BMC Gastroenterology (2018)