Abstract
Recently, we reported that a periodontopathic pathogen, Porphyromonas gingivalis (P. gingivalis), infection induced neointimal hyperplasia with enhanced expression of monocyte chemoattractant protein (MCP)-1 after arterial injury in wild-type mice. Toll-like receptor (TLR) 4 is known to be a key receptor for virulence factors of P. gingivalis. The aim of this study is to assess the hypothesis that TLR4 has a critical role in periodontopathic bacteria-induced neointimal formation after an arterial injury. Wild-type and TLR4-deficient mice were used in this study. The femoral arteries were injured, and P. gingivalis or vehicle was injected subcutaneously once per week. Fourteen days after arterial injury, murine femoral arteries were obtained for histopathological and immunohistochemical analyses. The anti-P. gingivalis IgG levels in P. gingivalis-infected groups were significantly increased compared with the anti-P. gingivalis IgG levels of the corresponding non-infected groups in both wild-type and TLR4-deficient mice. TLR4 deficiency negated P. gingivalis-induced neointimal formation compared with that observed in wild-type mice and reduced the number of MCP-1 positive cells in the neointimal area. We conclude that P. gingivalis infection may promote neointimal formation after an arterial injury through TLR4 signaling.
Similar content being viewed by others
Introduction
Percutaneous coronary intervention is a well-established therapy in coronary artery disease; however, the resulting mechanical damage to the vascular wall causes neointimal hyperplasia and blood vessel remodeling.1, 2 Upregulation of monocyte chemoattractant protein (MCP)-1 gene expression after coronary angioplasty induces neointimal hyperplasia and recruits monocyte and tissue macrophages to the arterial wall.3 Medical treatment with neutralizing MCP-1 antibody has resulted in a remarkable reduction of neointimal formation in a rat model of carotid injury.4
Periodontitis is an inflammatory disease that leads to the destruction of tooth-supporting tissue. The pathogen Porphyromonas gingivalis (P. gingivalis) is a major cause of human periodontitis.5 Toll-like receptors (TLRs) are a group of pattern recognition receptors that mediate the innate host response to microbial pathogens.6, 7 It is well known that P. gingivalis is recognized by TLR4; P. gingivalis lipopolysaccharides (LPS) treatment of gingival fibroblasts upregulates the expression of MCP-1 and nuclear factor-κB (NF-κB) via TLR4.8 Recent studies have shown that periodontal pathogens contribute to the pathogenesis of arterial diseases.9, 10 Periodontal pathogens may have a role in the development and progression of atherosclerosis, leading to cardiovascular disease.11, 12 We previously showed that P. gingivalis infection induced neointimal hyperplasia after arterial injury13 and that TLR2 deficiency suppressed the progression of the neointimal formation.14 However, the role of TLR4 blockade in neointimal formation accelerated by P. gingivalis infection after wire injury remains to be elucidated.
TLR4 is known to have a pivotal role in the progression of vascular remodeling.15 Bai et al. also showed that the expression of TLR4 protein increased in balloon-injured arteries compared with uninjured arteries. The inhibition of IL-1 receptor-associated kinase (IRAK) 1 and IRAK4, which were activated by TLR4, attenuated neointimal formation. Furthermore, the IRAK1/4 inhibitor suppressed the activation of the TLR4-mediated NF-κB pathway in vivo and in vitro.16 However, the mechanism by which periodontal bacterial infection accelerates neointimal formation through TLR4 after arterial injury has not yet been elucidated. On the basis of these facts, the aim of this study is to investigate the involvement of TLR4 in periodontopathic bacteria-induced neointimal formation after arterial injury.
Materials and methods
Animal protocol
Male C57BL/6 mice (wild-type; WT, 7 weeks, 20–25 g) were obtained from Japan Clea Co (Tokyo, Japan). Male TLR4 knockout (TLR4KO) mice in a C57BL/6 background were obtained from Oriental Yeast Co (Tokyo, Japan). The mouse strains were confirmed by genotyping, and age-matched mouse groups were used for experiments. The experimental procedures described here were approved by the Animal Welfare Committee and performed in accordance with the Animal Care Standards of Tokyo Medical and Dental University.
Bacterial preparation
P. gingivalis strain A7A1-28 was grown on blood agar plates in an anaerobic chamber with 85% N2, 5% H2 and 10% CO2. After incubation at 37 °C for 2–3 days, the bacteria were inoculated into a peptone yeast extract and incubated for a further week. The bacterial concentration was standardized to 108 colony forming units (CFUs) ml−1. The levels of anti-P. gingivalis-specific IgG in the plasma were determined by an enzyme-linked immunosorbent assay, as previously described.17
Chamber model
Coil-shaped subcutaneous chambers were prepared from 0.5 mm stainless-steel wire and surgically implanted in the subcutaneous tissue of the back region of each mouse. During the period before inoculation, the outer incision healed completely, and the chambers became encapsulated by a thin-vascularized layer of fibrous connective tissue. Fourteen days after implantation, mice were inoculated with 0.1 ml of a suspension of P. gingivalis in phosphate-buffered saline. The non-infected group was only inoculated with phosphate-buffered saline. Mice were killed 14 days after arterial injury, and plasma was separated from blood obtained by retro-orbital bleeding.
Wire injury model
In this study, we modified an arterial injury model.18 Briefly, the femoral artery was looped and tied off with 6-0 silk sutures for temporary vascular control during the procedure. A transverse arteriotomy was made, and a flexible angioplasty guidewire (a curved 350-μm polished copper wire) was introduced and advanced 1 cm. Endothelial denudation of the artery was performed by withdrawal of the wire; three passes were made along the artery.19, 20 A sham operation (no wire injury) was also performed. Both WT and TLR4KO mice were divided into two groups: those injured by arterial surgery and inoculated with live P. gingivalis (0.1 ml of 108 CFU ml−1) and those injured by arterial surgery and inoculated with vehicle containing diluted medium (0.1 ml). Sham-operated vessels (no wire injury) with P. gingivalis infection in WT and TLR4KO mice were used as controls. Subcutaneous injections were performed once per week for 14 days. Fourteen days later, the mice underwent laparotomy and dissection.19 Body weight was measured just before killing.21 Figure 1 shows the time schedule of this study.
Histological and morphometrical analyses
Histopathological analyses were performed as previously described.18 The sections were stained with Elastica van Gieson (EvG). Complete transverse sections of arteries ~3 mm in length were obtained.19 The persons who selected and measured the histological sections were blinded with respect to the intervention. The thickness of the intima, media and lumen within a cross-section of the artery in the slides stained with EvG was calculated using Image-Pro Express (Media Cybernetics, Silver Spring, MD, USA; n=6–10) software. The neointimal and medial areas of at least six sections per artery were measured. Some samples were excluded from statistical analysis when they included massive thrombus formation.
Immunohistochemistry
For immunohistochemical staining, anti-MCP-1 antibody and anti-CD31 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used (n=5, each). The number of immunohistochemically positive cells was counted per artery by a researcher who was blinded to the treatments of animals.
Statistical analysis
All data are expressed as the mean±s.e.m. An analysis of variance combined with the Kruskal–Wallis test was used to compare all groups. The differences between two selected groups were analyzed by Mann–Whitney’s U-test. Statistical significance was accepted at P<0.05.
Results
Quantification of antibacterial antibodies
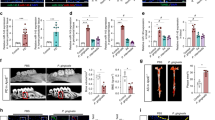
The effects of the repeated injection of P. gingivalis or vehicle on plasma levels of anti-P. gingivalis IgG were determined using enzyme-linked immunosorbent assay 14 days after injury in WT and TLR4KO mice. In WT mice, the anti-P. gingivalis IgG level of the infected group (n=8) was significantly higher than that of the non-infected group (n=8). In TLR4KO mice, the anti-P. gingivalis IgG level of the infected group (n=6) was also significantly higher than that of the non-infected group (n=6; Figure 2).
The levels of anti-P. gingivalis IgG. In WT and TLR4KO mice, the effects of repeated injection of P. gingivalis or vehicle on the plasma levels of anti-P. gingivalis IgG were determined. Plasma samples were obtained from the non-infected group and the P. gingivalis-infected group 2 weeks after injury. The levels of anti-P. gingivalis IgG are expressed as the means±s.e.m. *P<0.05, significant difference compared with the non-infected group.
Quantitative analysis of intimal thickening after wire injury
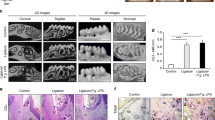
Areas of the vascular wall were quantitatively analyzed in the non-infected mice and the P. gingivalis-infected mice 14 days after injury. In all of the vascular wall sections, internal and external elastic laminae were identifiable by EvG staining (Figure 3). In WT mice, the intima/media thickness ratio in the P. gingivalis-infected group (n=10) was significantly increased in comparison with the non-infected group (n=10). It is noteworthy that TLR4 deficiency negated P. gingivalis-induced neointimal formation (n=10) compared with the WT mice. In TLR4KO mice, there was no significant difference in the intima/media thickness ratio between non-infected mice (n=6) and P. gingivalis-infected mice (n=6). Sham-operated vessels in P. gingivalis-infected WT and TLR4KO mice showed no intimal thickening (Figures 3 and 4). The body weight showed no significant difference between the groups (Table 1).
Pathological findings of injured arteries. Representative EvG-stained arterial sections in WT mice (upper panels) and TLR4KO mice (lower panels) are shown. The left panels show arteries from non-infected, injured mice, middle panels are arteries from P. gingivalis-infected and injured mice and the right panels are P. gingivalis-infected and non-injured mice. Injured arteries in WT mice infected with P. gingivalis (b) showed significantly thickened intima, while the degree of thickening was less severe in the injured arteries of TLR4KO mice infected with P. gingivalis (e). Sham-operated vessels of WT (c) and TLR4KO (f) mice infected with P. gingivalis showed no intimal thickening. Scale bars, 50 μm. A full color version of this figure is available at the Hypertension Research journal online.
Quantitative results of neointimal formation. The graph indicates the quantitative intima/media thickness. The amount of thickened intima was comparable between the non-infected WT and non-infected TLR4KO mice. However, the amount of thickened intima in the injured artery from WT mice infected with P. gingivalis was significantly higher than that from P. gingivalis-infected TLR4KO mice. Results are expressed as the means±s.e.m. *P<0.05.
Immunohistochemistry
In WT mice, the P. gingivalis-infected group (n=5) showed more MCP-1 positive cells in the neointimal area compared with the non-infected group (n=5, P<0.05) 14 days after injury. Among the P. gingivalis-infected groups, TLR4KO mice significantly suppressed the number of MCP-1 positive cells (n=5, P<0.05) compared with the P. gingivalis-infected WT mice. In TLR4KO mice, the numbers of MCP-1 positive cells were comparable between the non-infected and P. gingivalis-infected groups (Figures 5 and 6). To identify reendothelialization after arterial injury, we performed CD31 immunohistochemistry. We showed that all analyzed injured arteries had completely reendothelialized by day 14; there was no significant difference in CD31 staining among the groups (Figure 7).
Immunohistochemical analysis. Representative images of immunohistochemical detection of MCP-1 are shown. (a) An artery from a non-infected WT mouse and (b) an artery from a P. gingivalis-infected WT mouse. (c) An artery from a non-infected TLR4KO mouse and (d) an artery from a P. gingivalis-infected TLR4KO mouse. Scale bars, 10 μm. A full color version of this figure is available at the Hypertension Research journal online.
Representative images of immunohistochemical detection of CD31 are shown. (a) An injured artery from a non-infected WT mouse and (b) an injured artery from a P. gingivalis-infected WT mouse. (c) An injured artery from a non-infected TLR4KO mouse and (d) an injured artery from a P. gingivalis-infected TLR4KO mouse. Scale bars, 10 μm. A full color version of this figure is available at the Hypertension Research journal online.
Discussion
Periodontitis has been reported to be a significant independent risk factor for peripheral vascular disease.22 Recently, we reported that P. gingivalis infection accelerated neointimal formation after arterial injury.13 It has also been reported that Chlamydia pneumonia infection induced neointimal formation after arterial injury.23 Inflammation after infection is now considered a factor that worsens cardiovascular disease.
This study showed that repeated injection of P. gingivalis upregulated the anti-P. gingivalis IgG titer in not only WT mice but also in TLR4KO mice, which concurs with the findings of a previous report.24 This means that P. gingivalis was recognized even in TLR4KO mice. Although P. gingivalis infection induced neointimal hyperplasia after arterial injury in WT mice, P. gingivalis-induced neointimal hyperplasia was suppressed in TLR4KO mice. Pi et al. revealed that increased TLR4 and proinflammatory cytokines were observed in wire injury-induced carotid neointima. The TLR4 deficiency protected the injured carotid artery from neointimal formation via suppression of reactive oxygen species.25 Zhang et al. also showed that increased TLR4 and proinflammatory cytokines were observed in wire injury-induced carotid neointima. They revealed that PPAR-gamma inhibited vascular smooth muscle cell proliferation and migration by suppressing TLR4-mediated inflammation and attenuated intimal hyperplasia after carotid injury.26 There are many virulence factors of P. gingivalis, such as LPS, fimbriae, gingipains, proteases and hemagglutinin.27, 28, 29 Previous studies indicated that P. gingivalis LPS triggered inflammatory pathways through the production of chemokines via the TLR4 pathway in human aortic endothelial cells30 and human gingival fibroblasts.31
MCP-1 is a principal factor in initiation and progression of neointimal formation.32 MCP-1 is well known to have a critical role in the pathogenesis of coronary restenosis after percutaneous coronary intervention (PCI). Oshima et al. demonstrated that MCP-1 production at stented coronary arterial sites is associated with an increased risk of restenosis after clinical stent implantation. Therefore, we examined serum MCP-1 levels after arterial injury.33 MCP-1 is also known to be a critical factor for vascular smooth muscle cell proliferation.34 P. gingivalis LPS induces MCP-1 gene expression in human gingival fibroblasts.35 MCP-1 production is activated by TLR ligands, such as LPS and bacterial lipoprotein.36 We have shown that P. gingivalis infection increased the number of MCP-1 positive cells in the neointimal area in WT mice.13 In this study, however, the P. gingivalis-infected TLR4KO mice significantly suppressed the number of MCP-1 positive cells in the neointimal area compared with the P. gingivalis-infected WT mice. TLR4 ligand stimulation induced MCP-1 production in murine macrophage-like cells.37 These findings suggest that P. gingivalis infection induced MCP-1 expression via TLR4 stimulation and promoted neointimal hyperplasia after arterial injury.
Studies have demonstrated that MCP-1 has an important role in the mobilization or recruitment of endothelial progenitor cells, which may have beneficial effects on endothelial repair after injury. Fujiyama et al. showed that MCP-1-activated bone marrow-derived CD34-/CD14+ monocyte lineage cells (BM-MLCs) adhered onto injured endothelium, differentiated into endothelial cell-like cells and inhibited neointimal hyperplasia. BM-MLCs can function as endothelial cell progenitors, which have the ability to adhere to injured endothelium in an MCP-1-dependent manner, leading to reendothelialization associated with inhibition of intimal hyperplasia.38
Lucas et al. revealed the effect of chemokine blockade on plaque growth induced by P. gingivalis infection after aortic balloon injury in mice. They used a virus-derived anti-inflammatory protein, M-T7, which binds a broad spectrum of C, CC and CXC chemokines. Although P. gingivalis infection significantly increased monocyte invasion and arterial plaque growth after balloon injury, M-T7 treatment significantly blocked the pathological changes by modifying expression of TLR4. The results suggest a central role for chemokine-mediated inflammation after arterial injury in P. gingivalis-infected mice.39 Because sham-operated vessels with P. gingivalis-infected WT and TLR4KO mice showed no intimal thickening in our study, P. gingivalis itself has no effect on positive arterial remodeling.
We previously reported that a study of TLR2KO mice showed similar results to this TLR4KO study.14 Although we cannot compare these results directly, the reduced rate of intimal thickening seems to be comparable. Because signal cascades from these TLRs and from MCP-1 via NF-κB are related, similarities may exist between studies of different TLRKO mice; however, these may not be the only signaling cascades that impact intimal thickening.
Because reendothelialization after arterial injury is an important pathological phenomenon, we performed CD31 immunohistochemistry in this study.40 We showed that there was no difference in CD31 staining among the groups on day 14. Thus, examination of vessels at earlier time points may clarify the role of reendothelialization in arterial remodeling in P. gingivalis-infected mice with a TLR4 deficiency.
In summary, P. gingivalis induced neointimal hyperplasia after arterial injury in WT mice; however, TLR4KO mice suppressed neointimal hyperplasia induced by P. gingivalis after arterial injury. This suggests that P. gingivalis-induced neointimal hyperplasia is mediated by TLR4 signaling.
References
Yamamoto Y, Watari Y, Brydun A, Yoshizumi M, Akishita M, Horiuchi M, Chayama K, Oshima T, Ozono R . Role of the angiotensin II type 2 receptor in arterial remodeling after wire injury in mice. Hypertens Res 2008; 31: 1241–1249.
Haude M, Konorza TF, Kalnins U, Erglis A, Saunamaki K, Glogar HD, Grube E, Gil R, Serra A, Richardt HG, Sick P, Erbel R . Heparin-coated stent placement for the treatment of stenoses in small coronary arteries of symptomatic patients. Circulation 2003; 107: 1265–1270.
Stark VK, Hoch JR, Warner TF, Hullett DA . Monocyte chemotactic protein-1 expression is associated with the development of vein graft intimal hyperplasia. Arterioscler Thromb Vasc Biol 1997; 17: 1614–1621.
Furukawa Y, Matsumori A, Ohashi N, Shioi T, Ono K, Harada A, Matsushima K, Sasayama S . Anti-monocyte chemoattractant protein-1/monocyte chemotactic and activating factor antibody inhibits neointimal hyperplasia in injured rat carotid arteries. Circ Res 1999; 84: 306–314.
Socransky SS, Smith C, Haffajee AD . Subgingival microbial profiles in refractory periodontal disease. J Clin Periodontol 2002; 29: 260–268.
Lien E, Ingalls RR . Toll-like receptors. Crit Care Med 2002; 30: S1–11.
Kawai T, Akira S . Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011; 34: 637–650.
Wang PL, Ohura K . Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors. Crit Rev Oral Biol Med 2002; 13: 132–142.
Yu KM, Inoue Y, Umeda M, Terasaki H, Chen ZY, Iwai T . The periodontal anaerobe Porphyromonas gingivalis induced platelet activation and increased aggregation in whole blood by rat model. Thromb Res 2011; 127: 418–425.
Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ . Identification of periodontal pathogens in atheromatous plaques. J Periodontol 2000; 71: 1554–1560.
Gibson FC 3rd, Hong C, Chou HH, Yumoto H, Chen J, Lien E, Wong J, Genco CA . Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation 2004; 109: 2801–2806.
Li L, Messas E, Batista EL Jr, Levine RA, Amar S . Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model. Circulation 2002; 105: 861–867.
Kobayashi N, Suzuki JI, Ogawa M, Aoyama N, Hanatani T, Hirata Y, Nagai R, Izumi Y, Isobe M . Porphyromonas gingivalis accelerates neointimal formation after arterial injury. J Vasc Res 2012; 49: 417–424.
Kobayashi N, Suzuki J, Ogawa M, Aoyama N, Komuro I, Izumi Y, Isobe M . Porphyromonas gingivalis promotes neointimal formation after arterial injury through toll-like receptor 2 signaling. Heart Vessels 2014; 29: 542–549.
Nakashima T, Umemoto S, Yoshimura K, Matsuda S, Itoh S, Murata T, Fukai T, Matsuzaki M . TLR4 is a critical regulator of angiotensin II-induced vascular remodeling: the roles of extracellular SOD and NADPH oxidase. Hypertens Res 2015; 38: 649–655.
Bai S, Li D, Zhou Z, Cao J, Xu T, Zhang X, Wang Y, Guo J, Zhang Y . Interleukin-1 receptor-associated kinase 1/4 as a novel target for inhibiting neointimal formation after carotid balloon injury. J Atheroscler Thromb 2015; 22: 1317–1337.
Kojima T, Yano K, Ishikawa I . Relationship between serum antibody levels and subgingival colonization of Porphyromonas gingivalis in patients with various types of periodontitis. J Periodontol 1997; 68: 618–625.
Suzuki J, Ogawa M, Muto S, Yamaguchi Y, Itai A, Isobe M . The effects of pharmacological PAI-1 inhibition on thrombus formation and neointima formation after arterial injury. Expert Opin Ther Targets 2008; 12: 783–794.
Inagaki H, Suzuki J, Ogawa M, Taniyama Y, Morishita R, Isobe M . Ultrasound-microbubble-mediated NF-kappaB decoy transfection attenuates neointimal formation after arterial injury in mice. J Vasc Res 2006; 43: 12–18.
Suzuki J, Ogawa M, Takayama K, Taniyama Y, Morishita R, Hirata Y, Nagai R, Isobe M . Ultrasound-microbubble-mediated intercellular adhesion molecule-1 small interfering ribonucleic acid transfection attenuates neointimal formation after arterial injury in mice. J Am Coll Cardiol 2010; 55: 904–913.
Sata M, Maejima Y, Adachi F, Fukino K, Saiura A, Sugiura S, Aoyagi T, Imai Y, Kurihara H, Kimura K, Omata M, Makuuchi M, Hirata Y, Nagai R . A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. J Mol Cell Cardiol 2000; 32: 2097–2104.
Mendez MV, Scott T, LaMorte W, Vokonas P, Menzoian JO, Garcia R . An association between periodontal disease and peripheral vascular disease. Am J Surg 1998; 176: 153–157.
Pislaru SV, Van Ranst M, Pislaru C, Szelid Z, Theilmeier G, Ossewaarde JM, Holvoet P, Janssens S, Verbeken E, Van de Werf FJ . Chlamydia pneumoniae induces neointima formation in coronary arteries of normal pigs. Cardiovasc Res 2003; 57: 834–842.
Hayashi C, Madrigal AG, Liu X, Ukai T, Goswami S, Gudino CV, Gibson FC 3rd, Genco CA . Pathogen-mediated inflammatory atherosclerosis is mediated in part via Toll-like receptor 2-induced inflammatory responses. J Innate Immun 2010; 2: 334–343.
Pi Y, Zhang LL, Li BH, Guo L, Cao XJ, Gao CY, Li JC . Inhibition of reactive oxygen species generation attenuates TLR4-mediated proinflammatory and proliferative phenotype of vascular smooth muscle cells. Lab Invest 2013; 93: 880–887.
Zhang LL, Gao CY, Fang CQ, Wang YJ, Gao D, Yao GE, Xiang J, Wang JZ, Li JC . PPARγ attenuates intimal hyperplasia by inhibiting TLR4-mediated inflammation in vascular smooth muscle cells. Cardiovasc Res 2011; 92: 484–493.
Holt SC, Kesavalu L, Walker S, Genco CA . Virulence factors of Porphyromonas gingivalis. Periodontol 2000 1999; 20: 168–238.
Grenier D, La VD . Proteases of Porphyromonas gingivalis as important virulence factors in periodontal disease and potential targets for plant-derived compounds: a review article. Curr Drug Targets 2011; 12: 322–331.
Belanger M, Kozarov E, Song H, Whitlock J, Progulske-Fox A . Both the unique and repeat regions of the Porphyromonas gingivalis hemagglutin A are involved in adhesion and invasion of host cells. Anaerobe 2011; 18: 128–134.
Yumoto H, Chou HH, Takahashi Y, Davey M, Gibson FC 3rd, Genco CA . Sensitization of human aortic endothelial cells to lipopolysaccharide via regulation of Toll-like receptor 4 by bacterial fimbria-dependent invasion. Infect Immun 2005; 73: 8050–8059.
Herath TD, Darveau RP, Seneviratne CJ, Wang CY, Wang Y, Jin L . Tetra- and penta-acylated lipid A structures of Porphyromonas gingivalis LPS differentially activate TLR4-mediated NF-kappaB signal transduction cascade and immuno-inflammatory response in human gingival fibroblasts. PLoS One 2013; 8: e58496.
Schober A . Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol 2008; 28: 1950–1959.
Oshima S, Ogawa H, Hokimoto S, Nakamura S, Noda K, Saito T, Soejima H, Takazoe K, Ishibashi F, Yasue H . Plasma monocyte chemoattractant protein-1 antigen levels and the risk of restenosis after coronary stent implantation. Jpn Circ J 2001; 65: 261–264.
Kurauchi-Mito A, Ichihara A, Bokuda K, Sakoda M, Kinouchi K, Yaguchi T, Yamada T, Sun-Wada GH, Wada Y, Itoh H . Significant roles of the (pro)renin receptor in integrity of vascular smooth muscle cells. Hypertens Res 2014; 37: 830–835.
Watanabe A, Takeshita A, Kitano S, Hanazawa S . CD14-mediated signal pathway of Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Infect Immun 1996; 64: 4488–4494.
Pahl HL . Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999; 18: 6853–6866.
Masuda T, Deng X, Tamai R . Mouse macrophages primed with alendronate down-regulate monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1alpha (MIP-1alpha) production in response to Toll-like receptor (TLR) 2 and TLR4 agonist via Smad3 activation. Int Immunopharmacol 2009; 9: 1115–1121.
Fujiyama S, Amano K, Uehira K, Yoshida M, Nishiwaki Y, Nozawa Y, Jin D, Takai S, Miyazaki M, Egashira K, Imada T, Iwasaka T, Matsubara H . Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res 2003; 93: 980–989.
Lucas AR, Verma RK, Dai E, Liu L, Chen H, Kesavalu S, Rivera M, Velsko I, Ambadapadi S, Chukkapalli S, Kesavalu L . Myxomavirus anti-inflammatory chemokine binding protein reduces the increased plaque growth induced by chronic Porphyromonas gingivalis oral infection after balloon angioplasty aortic injury in mice. PLoS One 2014; 9: e111353.
Fukuda D, Enomoto S, Shirakawa I, Nagai R, Sata M . Fluvastatin accelerates re-endothelialization impaired by local sirolimus treatment. Eur J Pharmacol 2009; 612: 87–92.
Acknowledgements
We thank Ms. Noriko Tamura and Ms. Yasuko Matsuda for their excellent technical assistance. This work was supported by Grants-in-Aid for Scientific Research No. 21390553 and No. 23890057.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kobayashi, N., Suzuki, Ji., Aoyama, N. et al. Toll-like receptor 4 signaling has a critical role in Porphyromonas gingivalis-accelerated neointimal formation after arterial injury in mice. Hypertens Res 39, 717–722 (2016). https://doi.org/10.1038/hr.2016.58
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.58