Abstract
This open-label study investigated the long action of bisoprolol compared with metoprolol CR/ZOK for controlling the mean dynamic heart rate (HR) and blood pressure (BP) in patients with mild-to-moderate primary hypertension. Patients from seven centers in China were treated with either bisoprolol 5 mg or metoprolol CR/ZOK 47.5 mg once daily for 12 weeks. The primary end points were the mean dynamic HR reduction and the mean dynamic diastolic BP (DBP) control in the last 4 h of the treatment period. Secondary end points included ambulatory monitoring of the BP and HR, safety and compliance. A total of 186 patients, with 93 patients in each group, were enrolled and analyzed. In the last 4 h of the treatment period, patients receiving bisoprolol demonstrated a significantly greater reduction in the mean dynamic HR compared with patients receiving metoprolol CR/ZOK (least squares means (LSmeans) of difference: −3.79 b.p.m.; 97.5% confidence interval (CI): −7.45, −0.14; P=0.0202). Furthermore, in the last 4 h of the treatment period, bisoprolol demonstrated non-inferiority vs. metoprolol CR/ZOK in lowering the mean dynamic DBP (LSmeans of difference: −1.00; 97.5% CI: −4.79, 2.78; P=0.5495). Bisoprolol further significantly lowered the 24-h mean ambulatory, mean daytime and mean nighttime HR. The overall adverse event rate was similar between the two groups. Noncompliance was reported in 3 (3.53%) and 6 (7.32%) patients in the bisoprolol and metoprolol CR/ZOK groups, respectively. In conclusion, bisoprolol provided superior dynamic HR reduction and non-inferior dynamic BP reduction vs. metoprolol CR/ZOK in patients with mild-to-moderate hypertension. No new safety concerns were found.
Similar content being viewed by others
Introduction
Hypertension is a common health problem worldwide. In China, it was estimated that hypertension affected 200 million people in 2010, meaning 1 in 6 individuals had hypertension.1 Hypertension is a widely recognized risk factor for cardiovascular (CV) diseases, such as coronary heart disease and stroke. Adequate hypertension control can reduce the incidence of heart failure by 50%.2, 3
The sympathetic nervous system has an important role in blood pressure (BP) regulation, especially in the development and progression of hypertension and target organ damage. β-Blockers are commonly used antihypertensives that inhibit normal sympathetic effects through binding to β-adrenoceptors and blocking the binding of norepinephrine and epinephrine to these receptors.4 Various European and Chinese guidelines recommend β-blockers as one of the five classes of antihypertensive drugs for the initial and long-term maintenance treatment of hypertension.1, 5, 6 Both bisoprolol fumarate (Concor) and Metoprolol controlled release/zero-order kinetic tablets (metoprolol CR/ZOK) are commonly used β blockers in the treatment of hypertension. However, they have different pharmacodynamic and pharmacokinetic properties.7, 8, 9, 10
Both bisoprolol and metoprolol CR/ZOK are effective in lowering BP and heart rate (HR) in patients with mild-to-moderate hypertension.7, 8, 9, 10 Bisoprolol has a high oral bioavailability (up to 90%) and a long elimination half-life of 9–12 h in healthy subjects, which leads to a long duration of action that allows for once-daily oral administration.8 In addition, bisoprolol is a highly selective β1-blocker with a selectivity of 75:1 for β1 and β2 adrenergic receptors.11 Metoprolol CR/ZOK is a moderately selective β1-blocker with a selectivity of 20:1 for β1 and β2 adrenergic receptors.11 In addition, metoprolol CR/ZOK is a controlled-release formulation to release metoprolol succinate at an almost constant rate for 20 h.7, 12
Reportedly, 24-h BP variability is an independent risk factor for target organ damage and is possibly associated with increased CV events in the morning during the first few hours after waking.13 Because of the circadian pattern of BP, it has been suggested that such early morning BP surges may contribute to CV events such as non-embolic strokes and myocardial infarction, because the peak incidences of these CV events coincide with early morning rapid BP surges.13, 14 Therefore, complete BP control over the entire dosing period, including the early morning hours, is important.
There have been very few studies comparing efficacies, especially the long-acting BP control effects of these two long-acting regimens.9, 15, 16, 17 It has been reported that elevated resting HR was correlated with CV mortality and was an independent risk factor for CV diseases.18, 19, 20, 21 The European Society of Hypertension and the European Society of Cardiology stated that HR independently predicts CV morbidity or fatal events in several conditions, including hypertension.22 In addition, the coexistence of hypertension and high HR has been reported to be a predictor of a high incidence of stroke and coronary heart disease,23 and the risk of albuminuria in patients with type 2 diabetes was also positively associated with an increase of 1 beat per minute (b.p.m.) for morning HR and 1 mm Hg for morning systolic BP.24 Therefore, HR control should be an important consideration in choosing a proper 24-h long-acting medication for patients with hypertension. This phase IV, open-label clinical study aimed to compare the 24-h BP control and HR reduction capacity of bisoprolol and metoprolol CR/ZOK by testing their efficacies in lowering BP and HR during the last 4 h of a 12-week treatment period.
Methods
Patients
This is a randomized, multicenter, parallel, open-label clinical study. The trial was performed in seven hospitals in China from December 2011 to April 2014. Key inclusion criteria included: (1) patients with mild-to-moderate hypertension (World Health organization (WHO) stages I and II: mild hypertension—systolic BP (SBP) of 140–159 mm Hg and diastolic BP (DBP) of 90–99; moderate hypertension—SBP 160–179 mm Hg and DBP 100–109 mm Hg) who had not received antihypertensive drugs before their screening visit; (2) patients with mild hypertension who had used antihypertensive drugs before their screening visit but who could accept a 2-week washout to eliminate the residual effect of previously used antihypertensive drugs; and (3) patients between 18 and 70 years of age who had a clinical resting HR⩾70 b.p.m.
Key exclusion criteria included: (1) patients with previously treated moderate hypertension; and (2) patients with secondary hypertension, concomitant coronary heart disease, concomitant acute or chronic heart failure, a cerebrovascular event within 6 months of the study or hepatic or renal impairment as judged by local laboratory standards. Patients working night shifts were also excluded.
The trial was conducted in compliance with the ICH-Good Clinical Practice (ICH-GCP E6, 1996) and the Declaration of Helsinki (2013). Informed consent was obtained from each patient. This study was registered at clinicaltrials.gov (NCT01508325).
Study schedule and treatment
Baseline information was collected from eligible patients and they received physical and laboratory examinations at their screening visit (week −4). Patients with mild hypertension who had antihypertensive treatment before their screening visit had a 2-week washout period to eliminate the residual effects of the previous treatment.
At Visit 1 (week 0), patients were randomized to receive bisoprolol (bisoprolol fumarate tablet, Merck Serono KGaA, Darmstadt, Germany) 5 mg or metoprolol CR/ZOK (AstraZeneca, Mölndal, Sweden) 47.5 mg every day. Treatment doses were uptitrated every 4 weeks according to the schedule of Figure 1 if the patients had an SBP>140 mm Hg and/or DBP>90 mm Hg at one of their subsequent visits (weeks 4, 8 and/or 12). The whole study period lasted for 12 weeks (Figure 1). At week 12, patients underwent a laboratory examination and a physical examination that included vital signs and ambulatory BP monitoring (ABPM).
Because it was not possible to determine the number of patients enrolled at each center in advance, central randomization was performed for all patients rather than local randomization at each center. A central randomization table was prepared, and each center was provided with a copy of part of the central randomization table and sealed envelopes containing a treatment assignment card before the study began. Eligible patients were successively added to treatment groups according to the randomization table and the envelope they randomly received from the physicians at each center.
End points
The primary efficacy end points were the differences in changes in the mean dynamic DBP and the dynamic HR as measured by ABPM in the last 4 h at the end of the 12-week treatment period between the two groups. Secondary efficacy end points included differences in the change of other BP parameters, including the mean ambulatory SBP in the last 4 h, the mean ambulatory 24-h SBP and DBP, the mean ambulatory daytime and nighttime SBP and DBP and the BP response rate as well as differences in the change of other HR parameters, including the mean ambulatory 24-h HR, the mean ambulatory daytime and nighttime HR and the HR response rates. A BP response was defined as DBP⩽90 mm Hg or a ⩾10 mm Hg decrease in DBP from baseline. Patients who had ⩾10% decrease in HR were considered to have a HR response. The ABPM evaluators were blinded when they assessed patient’s ABPM.
Safety end points included adverse events (AEs), vital signs, BP, HR, laboratory examination, and electrocardiogram examination. Adverse events were evaluated during every visit as the randomization was implemented. In addition to ABPM at baseline and week 12, BPs were also measured using a regular sphygmomanometer at each visit.
Treatment compliance was measured at visits 2–4. Compliance (%) was calculated as (total number of used drugs/total number of drugs that should be used) × 100. Total number of drugs that should be used was calculated based on the actual treatment duration of each subject. Treatment compliance that was <80% was considered to be non-compliant. To check patients’ medical compliance, empty drug boxes that had been used between two visits were returned, and the amount of study drug used between the two visits was counted and recorded.
Method of ABPM
Baseline and week 12 dynamic DBP, SBP and HR were monitored by 24-h ABPM. The time point when patients started wearing, or when they removed, the dynamic BP monitor and whether it was a workday or a holiday when the ABPM measurements were performed were recorded for both ABPM measurements. Baseline measurements were made within 4 weeks before the first administration of the treatment drug. If there was a washout period, a baseline measurement was made after the washout period. However, baseline ABPMs for all patients were collected before randomization. For the second ABPM at week 12, subjects were required to take the study drug at 0800 hours±10 min on the first day to start the 24-h ABPM and also the next day to assure the same last 4-h period of the treatment was used for all of the patients. For most patients, whether to perform the second ABPM on a workday or a holiday was consistent with their first ABPM. For an example, if the baseline ABPM of a patient started at 0800 hours on Thursday and ended at 0800 hours on Friday, the second ABPM for this patient was started at the same time on a workday. The ABPM device used in this study was manufactured by Spacelabs Healthcare (Snoqualmie, WA, USA) and verified by British Hypertension Society, Association for the Advancement of Medical Instrumentation and/or European Society of Hypertension protocols. In the current study, ABPM measured BP three times per hour during the daytime and hourly during the nighttime. Only data with ⩾80% valid data were used for analysis. Finally, daytime was defined as 0600–2200 hours, and nighttime was defined as 2200–0600 hours.
Calculation of sample size
A normal distribution was expected from the changes of the mean dynamic HR and the mean dynamic DBP. In addition, it was further assumed that, compared with the metoprolol CR/ZOK, bisoprolol would have a 2-b.p.m. more decrease in the mean HR with an s.d. of 4 b.p.m. in both arms and a 2-mm Hg less in the change of the mean dynamic DBP with an s.d. of 4 mm Hg in both arms. With a population error rate of 0.05 and a dropout rate of 20%, it was estimated that a sample size of 184 cases would provide >85% power for each test.
Statistical analysis
The efficacy statistical analyses were based on the intent-to-treat (ITT) population, which included all randomized patients with last observation carried forward, and the per-protocol set (PPS), which included all subjects in the ITT set without major protocol violations during the study, was used for supportive analysis. Safety analyses were based on the safety population, which included all patients who received at least one dose of study drug during the study.
An analysis of covariance model was used for intergroup comparison of the primary end points with baseline mean DBP or baseline HR and also with gender as the covariates and group and center as the fixed effects. For calculation of the confidence interval (CI), least squares means (LSmeans) of the difference in change between the two groups (μ test−μ control) and a 97.5% CI were calculated based on the model. For comparison of changes in the mean DBP in the last 4 h of the treatment period between the bisoprolol vs. metoprolol CR/ZOK groups, the upper limit of the CI of differences in the change of the mean dynamic DBP was compared with a non-inferiority margin of 4 mm Hg. Statistical significance was accepted with a P value<0.025. Because this study included multiple end points, all results of statistical tests were adjusted based on multiple tests. Multiple tests were adjusted based on the Bonferroni method.
For secondary efficacy end points, except the BP and HR response rates, descriptive statistics was performed. For an intergroup comparison, a t-test was used. The Cochran–Mantel–Haenszel chi-square test stratified by center was used for intergroup comparison of the BP response rate and HR response rate after 12 weeks of treatment.
Various subgroup analyses were performed, including different dose groups for patients treated with a low dose (without dose adjustment), a medium dose (uptitration after Visit 1) and a high dose (uptitration after Visit 2), according to dose adjustment during treatment. Additionally, subgroup analyses were performed for HR, baseline BP, smoking, age, gender and circadian rhythm of BP. The same statistical analyses used in the primary and secondary end point analyses were performed.
Results
Patients
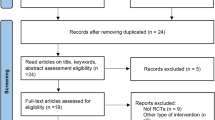
A total of 184 patients were expected, and 186 outpatients with mild-to-moderate hypertension (WHO stages I and II) from 7 hospitals in China were enrolled in the study. All patients were randomized (93 in the bisoprolol group and 93 in the metoprolol CR/ZOK group). Fifty-nine patients had protocol deviations. Four patients completed the washout period. Seventy-five patients in the bisoprolol and 72 in the metoprolol CR/ZOK groups finished the planned treatment (Figure 2). There were more men in the bisoprolol group than in the metoprolol CR/ZOK group (61.29% vs. 39.78%). All other demographic data, including vital signs, laboratory exam results and concomitant medications, were comparable between the two groups (Table 1). More patients in the bisoprolol group had a low dose treatment without uptitration (72%) than patients in the metoprolol CR/ZOK group (58%). The study drug dose distribution is also shown in Table 1. Overall, 3% of the patients in the bisoprolol and 5% in the metoprolol CR/ZOK groups had uptitration to a high-dose treatment (Table 2).
Primary end points
Analyses of primary end points were conducted for the ITT population. Both bisoprolol and metoprolol CR/ZOK lowered the mean dynamic HR in the last 4 h of the 12-week treatment period. Bisoprolol led to a significantly greater reduction vs. metoprolol CR/ZOK (−8.04 vs. −4.75 b.p.m., LSmeans of difference (97.5% CI)=−3.79 b.p.m. (−7.45, −0.14); P=0.0202) (Figure 3a; Table 3).
Changes in the mean heart rate and the mean dynamic diastolic blood pressure (DBP) in the last 4 h of the treatment period from baseline (intent-to-treat population (ITT)). (a) Change in the mean heart rate in the last 4 h of the treatment period from baseline. (b) Change in the DBP in the last 4 h of the treatment period from baseline.
Both bisoprolol and metoprolol CR/ZOK significantly lowered the mean dynamic DBP in the last 4 h of the 12-week treatment period (bisoprolol: −4.45 mm Hg, P=0.0014; metoprolol CR/ZOK: −3.39, P=0.0168). LSmeans of the difference in the change of mean dynamic DBP before and after treatment with bisoprolol and metoprolol CR/ZOK was −1.00 (97.5% CI: −4.79, 2.78), P=0.5495, and the upper limit of the 97.5% CI was less than the 4 mm Hg non-inferiority margin, which indicates that bisoprolol was non-inferior to metoprolol CR/ZOK in lowering the mean dynamic DBP (Figure 3b; Table 3).
The PPS results were consistent with the ITT results (Table 3).
Secondary end points
Our results for the ITT population showed that patients treated with bisoprolol had a greater mean 24-h ambulatory HR reduction than patients treated with metoprolol CR/ZOK (−8.46 vs. −3.24 b.p.m., P=0.0015). The mean ambulatory daytime and nighttime HR reductions (−9.60 vs. −3.85 b.p.m., P=0.0012; −4.72 vs. −1.50 b.p.m., P=0.0347, respectively) were also greater with bisoprolol vs. metoprolol CR/ZOK (Table 3).
For 24-h dynamic BP control, our results for the ITT population indicated that both drugs could lower mean ambulatory SBP in the last 4 h of the treatment period as well as the mean ambulatory 24-h BP, the mean ambulatory daytime BP and the mean ambulatory nighttime DBP and SBP (Table 3). No significant differences were noted between the two treatment groups (Table 3).
In addition, our results in the ITT population showed comparable BP response rates between the two treatment groups (bisoprolol: 86.67%; metoprolol CR/ZOK: 83.33%; P=0.5487, Figure 4a). Our data also showed that there were comparable HR response rates between the two treatment groups (bisoprolol 72.00%; metoprolol CR/ZOK: 55.56%; P=0.0527, Figure 4b).
PPS results for all of the secondary end points were consistent with the ITT results (Table 3).
Subgroup analysis
Subgroup analysis of the ITT population by dose group, baseline BP, smoking, age, gender and circadian rhythm of BP did not identify differences in the primary and secondary end points between the two treatment groups. Analysis by baseline HR showed that a greater mean dynamic HR reduction was associated with bisoprolol compared with metoprolol CR/ZOK in patients with a baseline clinical HR>80 b.p.m. (bisoprolol: −12.97 b.p.m.; metoprolol CR/ZOK: −6.13 b.p.m.; P=0.0281). The PPS results were consistent with the ITT results (data not shown).
Treatment compliance and safety
There were few patients who reported noncompliance during the study (3 (3.53%) patients in the bisoprolol group and 6 (7.32%) patients in the metoprolol CR/ZOK group). Overall, 81 (95.29) patients in the bisoprolol group and 76 (92.68%) patients in the metoprolol CR/ZOK group reported 80–120% compliance. One patient in the bisoprolol group reported >120% compliance.
A total of 19 patients (20.4%) in the bisoprolol group and 16 patients (17.2%) in the metoprolol CR/ZOK group reported AEs. Among these events, the AEs reported by nine patients in the bisoprolol group and six patients in the metoprolol CR/ZOK group were drug related (Supplementary Table S1).
Two patients reported two serious AEs (dizziness and transient ischemic attack) in the bisoprolol group, and dizziness was judged to be drug related by the investigator. No serious AEs were reported in the metoprolol CR/ZOK group (Supplementary Table S1).
Discussion
In this randomized, open-label clinical study, we compared a therapeutically comparable dose of metoprolol CR/ZOK to bisoprolol. In the last 4 h of the 12-week treatment period, bisoprolol led to a significantly greater reduction in the mean dynamic HR and a non-inferior reduction in the mean dynamic DBP and SBP. Thus the results indicate a comparable long-acting effect on BP control and a more potent long-acting effect on HR reduction associated with bisoprolol vs. metoprolol CR/ZOK. For daytime, nighttime and 24-h BP and HR reduction, bisoprolol also showed a significantly greater HR reduction and comparable BP control vs. metoprolol CR/ZOK. In the subgroup analysis, a significantly greater mean HR reduction was associated with bisoprolol in a subgroup of patients with a baseline clinical HR>80 b.p.m. Comparable HR and BP response rates were noted for the two medications. Both drugs had high compliance and no new safety concerns were noted in our study.
Both bisoprolol and metoprolol CR/ZOK are commonly used long-acting β-blockers for treating hypertension, and they could achieve long-acting effects through different pharmacokinetic and phymacokinetic mechanisms.7, 8, 9 The few existing studies comparing the efficacy and safety of bisoprolol and metoprolol provided inconclusive results.9, 15, 16, 17 The BISOMET study demonstrated that, 24 h after drug intake, bisoprolol was significantly more effective at lowering exercise SBP, DBP and HR as well as resting DBP and HR.9 However, a conventional metoprolol formulation, instead of the long-acting formulation, was used in that study. Our results were inconsistent with the results of the study by Kronig17 in that a significantly greater reduction in exercise HR and BP were associated with metoprolol CR/ZOK vs. bisoprolol 24 h after drug administration. This inconsistency could possibly be explained by the fact that Kronig17 tested exercise HR and BP 24 h after drug administration, whereas we tested the mean dynamic HR and BP in the last 4 h of the drug administration interval by ABPM. In addition, we want to postulate that ethnicity might have a role here. It has been reported that ethnicity differences may affect BP reduction responses to various antihypertensive β-blockers.25 One thing worth mentioning is that in the current study, we chose differences in mean dynamic DBP reduction instead of SBP reduction as a primary end point because the patients’ ages ranged from 18 to 70 years in our study with a mean age of 51.10±10.77 and 49.89±10.46 years for the bisoprolol and metoprolol CR/ZOK groups, respectively (Table 1). It has been reported that 24-h ambulatory DBP rather than SBP was a predominant risk factor for coronary complications in patients aged <50 years.26 Therefore, we wanted to focus our attention on DBP reduction in this study rather than SBP, which has been studied more often.
Our results showed that superior HR control was associated with bisoprolol vs. metoprolol CR/ZOK, which indicated stronger control overall. Residual HR control also occurred at the end of a dose interval, especially in patients with a baseline clinical HR>80 b.p.m. Elevated HR is often caused by an imbalance between an overactive sympathetic system and/or decreased vagal system,20 and it has been reported that elevated resting HR was correlated with CV mortality and was an independent risk factor for CV diseases.18, 19, 20, 21 The European Society of Hypertension and the European Society of Cardiology stated that HR independently predicts CV morbidity or fatal events in several conditions, including hypertension.22 Furthermore, HR reduction has been associated with a better prognosis, such as less mortality and less rehospitalization.18 When compared with daytime HR, nighttime HR acquires increased importance. It has been found that nighttime HR was a better predictor of CV events than daytime HR in hypertensive patients.27 This difference may be due to increased sympathetic activity, which may cause unfavorable hemodynamic effects that could accelerate atherosclerosis. Therefore, a high nighttime HR could better reflect mechanical stress-induced arterial injury.27 In fact, the sympathetic overactivity underlying elevated HR could also explain the reported association between elevated nighttime HR and BP, sleep apnea, body mass index and metabolic abnormalities.27 Thus, when choosing a 24-h long-action medication for hypertensive patients, HR control should be an important consideration. Bisoprolol therefore could be a preferable antihypertensive β-blocker for treating hypertension patients with increased sympathetic activity.
Increased sympathetic activity has an important role in hypertension pathogenesis. The better HR control observed in the bisoprolol group may be associated with a trend toward better BP control, especially during the nighttime. As we mentioned earlier, 24-h BP variability control is important. It has been reported that early morning hypertension was associated with stroke prognosis, and a greater degree of morning BP surge was shown to be an independent risk factor for stroke.28, 29, 30, 31 Nighttime BP control has also drawn much attention because it could be a better predictor of mortality and CV events, such as stroke, than daytime BP in patients with and without hypertension,30, 31 especially because there have been reports that an 8% or 10% increase in the night-to-day ratio of SBP was independently associated with CV morbidity, including stroke, among untreated older patients with systolic hypertension and also in the general population.30 There was evidence that bisoprolol could reduce left ventricular mass, preserve systolic function and improve diastolic function of the left ventricle in hypertensive subjects with left ventricular hypertrophy, which demonstrated that organ protection occurred in hypertensive patients treated with bisoprolol.32 It is possible that both the reduction of sympathetic neurotoxicity and the control of nighttime BP may contribute to these effects.
The fact that bisoprolol showed superior HR reduction and comparable DBP control vs. metoprolol CR/ZOK in the last 4 h of the daily-dosing period, which is a period when the circulating concentration of any drug is the lowest during the dosing period, was significant and meaningful. Early morning BP surges may contribute to CV events such as non-embolic strokes and myocardial infarction, because the peak incidence of these CV events coincide with early morning rapid BP surges.13, 14 Additionally, it has been reported that an increase of 5 b.p.m. in the home-measured morning HR was independently associated with a 17% increase in the risk of CV mortality.33 The risk of albuminuria in patients with type 2 diabetes was positively associated with an increase of 1 b.p.m. in morning HR and a 1 mm Hg increase in morning systolic BP.24 Therefore, BP and HR control over the entire dosing period, including the early morning hours, is extremely important, and according to our study, bisoprolol performed very well in this capacity.
One factor that could potentially affect our results on HR was the fact that there was a significantly higher percentage of male patients in the bisoprolol group vs. the metoprolol CR/ZOK group (P=0.0034). CV autonomic regulation is an important factor in cardiac mortality, and HR variability as well as baroreflex sensitivity were two commonly used methods for assessing this automatic regulation.34 It has also been reported that, in middle-aged women, baroreflex sensitivity and a low-frequency component of HR variability was attenuated compared with men, although the high-frequency component of HR was higher in women compared with men, which suggests that women with a lower baroreflex sensitivity before an acute ischemic event would be at greater risk of mortality when they experience the event.34 However, because analysis of covariance, including gender as one of the covariates, was used in our study to compare the changes in the mean HR during the last 4 h of treatment, we expected the effect of the different distributions of genders in the two treatment groups to be minimal.
Drug compliance is one important aspect of patient care, and a high drug compliance could result in good treatment efficacy if the right drug is selected. Both drugs had high drug compliance, and complying with the treatment regimen did not pose a problem for patients. Thus compliance should not have an important role in determining which one of these drugs should be used to treat a particular patient.
Both bisoprolol and metoprolol CR/ZOK were well tolerated, and they had a comparable overall AE rate and study-drug-related AE rate. No new safety concerns were raised in this study. Overall, our results on AEs were consistent with previous reports.6, 7, 8 Both of these drugs were safe and had few AEs. Therefore, both are proper choices for treating mild-to-moderate hypertension.
Limitations
This study investigated the efficacy of bisoprolol on HR and BP control compared with metoprolol CR/ZOK at the end of a 12-week treatment period. A long-term study is needed to confirm the effectiveness of the medication and its impact on prognosis. The study was further limited by the fact that it was an open-label study in which the doctors and patients were not blinded to their treatment. However, the ABPM evaluators were blinded when they assessed ABPMs from patients in the bisoprolol and metoprolol CR/ZOK groups. As such, the study can be considered an open-label but end point-blinded study, and the bias inherent in an open-label study could at least be substantially overcome, although we could not totally disregard the effect of patients’ expectations on the BP and HR end points. Another limitation of the study was the relatively high rate of protocol deviation, which led to a relatively small number of patients in the PPS (127 patients in the PPS vs. 186 patients in the ITT). In a non-inferiority test, analyses based on ITT and PPS are equally important, and to reach a robust conclusion, their results should be consistent.35 Despite the relatively small sample of our PPS, the fact that the results of our ITT and PPS analyses were consistent suggested that the results were robust.
Conclusions
Both bisoprolol and metoprolol CR/ZOK could have long-term action. When compared with metoprolol CR/ZOK, bisoprolol showed superior mean dynamic HR reduction in the last 4 h of the treatment period, especially in patients with a baseline HR⩾80 b.p.m. Bisoprolol demonstrated non-inferior dynamic BP control vs. metoprolol CR/ZOK in patients with mild-to-moderate hypertension. No new safety concerns were found.
References
Liu LS Writing Group of 2010 Guidelines for the Management of Hypertension. 2010 Guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi 2011; 39: 579–615.
Prugger C, Keil U, Wellmann J, de Bacquer D, de Backer G, Ambrosio GB, Reiner Z, Gaita D, Wood D, Kotseva K, Heidrich J EUROASPIRE III Study Group. Blood pressure control and knowledge of target blood pressure in coronary patients across Europe: results from the EUROASPIRE III survey. J Hypertens 2011; 29: 1641–1648.
Arnett DK . Transforming cardiovascular health through genes and environment: presidential address at the American Heart Association 2012 Scientific Sessions. Circulation 2013; 127: 2066–2070.
Grassi G, Bertoli S, Seravalle G . Sympathetic nervous system: role in hypertension and in chronic kidney disease. Curr Opin Nephrol Hypertens 2012; 21: 46–51.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Rydén L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA . 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34: 2159–2219.
Mancia G, Laurent S, Agabiti-Rosei E . Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009; 27: 2121–2158.
Plosker GL, Clissold SP . Controlled release metoprolol formulations. A review of their pharmacodynamic and pharmacokinetic properties, and therapeutic use in hypertension and ischaemic heart disease. Drugs 1992; 43: 382–414.
Lancaster SG, Sorkin EM . Bisoprolol. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in hypertension and angina pectoris. Drugs 1988; 36: 256–285.
Haasis R, Bethge H . Exercise blood pressure and heart rate reduction 24 and 3 h after drug intake in hypertensive patients following 4 weeks of treatment with bisoprolol and metoprolol: a randomized multicentre double-blind study (BISOMET). Eur Heart J 1987; 8 (Suppl M): 103–113.
Larochelle P, Tobe SW, Lacourcière Y . β-Blockers in hypertension: studies and meta-analyses over the years. Can J Cardiol 2014; 30: S16–S22.
Wellstein A, Palm D, Belz GG, Butzer R, Polsak R, Pett B . Reduction of exercise tachycardia in man after propranolol, atenolol and bisoprolol in comparison to beta-adrenoceptor occupancy. Eur Heart J 1987; 8 (Suppl M): 3–8.
Wikstrand J . Achieving optimal beta1-blockade with metoprolol CR/Zok. Basic Res Cardiol 2000; 95: I46–151.
Parati G, Faini A, Valentini M . Blood pressure variability: its measurement and significance in hypertension. Curr Hypertens Rep 2006; 8: 199–204.
Neutel JM, Alderman M, Anders RJ, Weber MA . Novel delivery system for verapamil designed to achieve maximal blood pressure control during the early morning. Am Heart J 1996; 132: 1202–1206.
Unzueta Montoya A, Unzueta A Jr, Ordóñez Toquero G, Villasis Keever MA, Cocoletzi López J, Medina Santillán R . Comparative study between bisoprolol and metoprolol, combined with hydrochlorothiazide, as antihypertensive therapy. Arch Inst Cardiol Mex 2000; 70: 589–595.
Metelitsa VI, Duda SG, Gorbunov VM, Buchner-Moll D, Deev AD, Vygodin VA, Filatova NP, Chel'dieva EIa, Shastun RS, Simonov DV . The antihypertensive effect of the new cardioselective prolonged-action beta-adrenoblocker bisoprolol compared with propranolol, metoprolol and placebo. Eksp Klin Farmakol 1995; 58: 32–34.
Kronig B . Influence of galenics on 24-h blood pressure control. Comparison of a new formulation of metoprolol versus bisoprolol in essential hypertension. Her Kreislauf 1990; 22: 224–229.
Caetano J, Delgado Alves J . Heart rate and cardiovascular protection. Eur J Intern Med 2015; 26: 217–222.
Palatini P, Thijs L, Staessen JA, Fagard RH, Bulpitt CJ, Clement DL, de Leeuw PW, Jaaskivi M, Leonetti G, Nachev C, O'Brien ET, Parati G, Rodicio JL, Roman E, Sarti C, Tuomilehto J Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Predictive value of clinic and ambulatory heart rate for mortality in elderly subjects with systolic hypertension. Arch Intern Med 2002; 162: 2313–2321.
Salles GF, Cardoso CR, Fonseca LL, Fiszman R, Muxfeldt ES . Prognostic significance of baseline heart rate and its interaction with beta-blocker use in resistant hypertension: a cohort study. Am J Hypertens 2013; 26: 218–226.
Kolloch R, Legler UF, Champion A, Cooper-Dehoff RM, Handberg E, Zhou Q, Pepine CJ . Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: findings from the INternational VErapamil-SR/trandolapril STudy (INVEST). Eur Heart J 2008; 29: 1327–1334.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F Task Force for the Management of Arterial Hypertension of the European Society of Hypertension and the European Society of Cardiology. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press 2014; 23: 3–16.
Zhong C, Zhong X, Xu T, Peng H, Li H, Zhang M, Wang A, Xu T, Sun Y, Zhang Y . Combined effects of hypertension and heart rate on the risk of stroke and coronary heart disease: a population-based prospective cohort study among Inner Mongolians in China. Hypertens Res 2015; 38: 883–888.
Ushigome E, Fukui M, Hamaguchi M, Tanaka T, Atsuta H, Ohnishi M, Tsunoda S, Yamazaki M, Hasegawa G, Nakamura N . Home-measured heart rate is associated with albuminuria in patients with type 2 diabetes mellitus: a post-hoc analysis of a cross-sectional multicenter study. Hypertens Res 2014; 37: 533–537.
Gupta AK, Poulter NR, Dobson J, Eldridge S, Cappuccio FP, Caulfield M, Collier D, Cruickshank JK, Sever PS, Feder G ASCOT. Ethnic differences in blood pressure response to first and second-line antihypertensive therapies in patients randomized in the ASCOT Trial. Am J Hypertens 2010; 23: 1023–1030.
Li Y, Wei FF, Thijs L, Boggia J, Asayama K, Hansen TW, Kikuya M, Björklund-Bodegård K, Ohkubo T, Jeppesen J, Gu YM, Torp-Pedersen C, Dolan E, Liu YP, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Mena L, Maestre GE, Filipovský J, Imai Y, O'Brien E, Wang JG, Staessen JA International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO) Investigators. Ambulatory hypertension subtypes and 24-hour systolic and diastolic blood pressure as distinct outcome predictors in 8341 untreated people recruited from 12 populations. Circulation 2014; 130: 466–474.
Palatini P, Reboldi G, Beilin LJ, Eguchi K, Imai Y, Kario K, Ohkubo T, Pierdomenico SD, Saladini F, Schwartz JE, Wing L, Verdecchia P . Predictive value of night-time heart rate for cardiovascular events in hypertension. The ABP-International study. Int J Cardiol 2013; 168: 1490–1495.
Hoshide S, Kario K . Early morning hypertension: a narrative review. Blood Press Monit 2013; 18: 291–296.
Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K . Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 2003; 107: 1401–1406.
Yano Y, Kario K . Nocturnal blood pressure, morning blood pressure surge, and cerebrovascular events. Curr Hypertens Rep 2012; 14: 219–227.
Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA . Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension 2008; 51: 55–61.
de Teresa E, González M, Camacho-Vázquez C, Tabuenca MJ . Effects of bisoprolol on left ventricular hypertrophy in essential hypertension. Cardiovas Drugs Ther 1994; 8: 837–843.
Hozawa A, Ohkubo T, Kikuya M, Ugajin T, Yamaguchi J, Asayama K, Metoki H, Ohmori K, Hoshi H, Hashimoto J, Satoh H, Tsuji I, Imai Y . Prognostic value of home heart rate for cardiovascular mortality in the general population. AJH 2004; 17: 1005–1010.
Huikuri HV, Pikkujämsä SM, Airaksinen KE, Ikäheimo MJ, Rantala AO, Kauma H, Lilja M, Kesäniemi YA . Sex-related differences in autonomic modulation of heart rate in middle-aged subjects. Circulation 1996; 94: 122–125.
Gupta SK . Non-inferiority clinical trials: practical issues and current regulatory perspective. Indian J Pharmacol 2011; 43: 371–374.
Acknowledgements
We thank all investigators, study teams and patients for their participation in this study. We especially want to thank Hai Deng from the First People’s Hospital of Yueyang and Weiwei Liu from the First Hospital of Changsha for their support. Medical writing assistance was provided by Xin Liu, PhD of Clinical Intelligence Consolidation and Application and funded by the Merck Serono, Beijing, China, an affiliate of Merck KGaA, Darmstadt, Germany. This study was sponsored by the Merck Serono, whose parent company, Merck KGaA, manufactured the study drug bisoprolol.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Hypertension Research website
Supplementary information
Rights and permissions
About this article
Cite this article
Yang, T., Jiang, Y., Hao, Y. et al. Comparison of bisoprolol to a metoprolol CR/ZOK tablet for control of heart rate and blood pressure in mild-to-moderate hypertensive patients: the CREATIVE study. Hypertens Res 40, 79–86 (2017). https://doi.org/10.1038/hr.2016.101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.101
Keywords
This article is cited by
-
The Combination of Beta-Blockers and ACE Inhibitors Across the Spectrum of Cardiovascular Diseases
Cardiovascular Drugs and Therapy (2023)
-
Effectiveness and Tolerability of Bisoprolol/Perindopril Single-Pill Combination in Patients with Arterial Hypertension and a History of Myocardial Infarction: The PRIDE Observational Study
Advances in Therapy (2023)
-
Therapeutic Properties of Highly Selective β-blockers With or Without Additional Vasodilator Properties: Focus on Bisoprolol and Nebivolol in Patients With Cardiovascular Disease
Cardiovascular Drugs and Therapy (2022)