Abstract
The aim of the present study is to examine the effects of the antihypertensive drug cilnidipine on glucose metabolism and adipocytokines, including adiponectin, in diet-induced obese (DIO) mice. The effects of cilnidipine on insulin sensitivity and the levels of adiponectin in DIO mice were examined after the mice had been treated with cilnidipine dissolved in water at a dose of 0.2 g l−1 for 14 days. As expected, treatment with cilnidipine decreased the systolic and diastolic blood pressures in DIO mice, compared with control mice (P<0.05 for each parameter). Cilnidipine treatment improved glucose and insulin sensitivity in DIO mice. In addition, cilnidipine treatment dramatically increased the level of adiponectin in white adipose tissue (P<0.05) and the circulating levels of total and high-molecular weight (HMW) adiponectin in DIO mice (P<0.01 for each parameter). Furthermore, the secretion of HMW adiponectin and the ratio of HMW adiponectin/total adiponectin were both increased after cilnidipine treatment. Finally, the secretion of adiponectin from adipocytes was increased after cilnidipine treatment. Taken together, these results indicate that cilnidipine improves insulin tolerance and adiponectin levels, especially high-molecular type adiponectin, in DIO mice.
Similar content being viewed by others
Introduction
Several common disorders, such as hyperglycemia and hypertension, are seen in individuals with metabolic syndrome.1 Metabolic disorders, such as diabetes and hypertension, are thought to be key to the simultaneous development of these common disorders in certain individuals, along with the associated development of cardiovascular and cerebrovascular disease.2
Previous studies demonstrated the presence of Ca2+ transport system and voltage-sensitive Ca2+ channels in adipocytes.3 In addition, abnormal cellular calcium handling, particularly elevations in cytosolic-free calcium concentrations, are involved in insulin resistance and hypertension.4 Antihypertensive drugs have been shown to have a therapeutic affect not only on blood pressure but also on other facets of metabolic disorders.5, 6 For instance, insulin sensitivity in patients with hypertension was improved after the administration of cilnidipine, a Ca channel blocker.7 Thus, cilnidipine might be useful for patients with hypertension and diabetes mellitus based on its effects on glucose metabolism. However, the detailed mechanisms by which cilnidipine functions to ameliorate the effects of abnormal glucose metabolism remain unclear.
Several adipocytokines have crucial roles in the regulation of glucose metabolism.8, 9 Adipocytokines including tumor necrosis factor-alpha (TNF-alpha), IL-6 and resistin, which are all secreted from white adipose tissue (WAT), are known to be involved in glucose metabolism.10, 11, 12 In addition, adiponectin and high-molecular weight (HMW) adiponectin have a number of vascular protective qualities, such as anti-diabetic, anti-inflammatory and anti-atherogenic effects.13, 14, 15, 16 Taken together, these findings suggest that adipocytokines regulate insulin sensitivity and may be related to the pathogenesis of diabetes.
In the present study, therefore, we focused on the effects of cilnidipine, an antihypertensive drug, on glucose metabolism and adipocytokine levels in diet-induced obesity. We hypothesized that cilnidipine might affect glucose metabolism as well as the production and/or secretion of adipocytokines. To address this issue, we used diet-induced obese (DIO) diabetic mice to investigate the effects of cilnidipine on metabolic parameters, such as glucose and insulin levels during a glucose/insulin tolerance test (ITT), and the levels of adipocytokines, such as TNF-alpha, IL-6, resistin and adiponectin, in WAT and serum.
Methods
Animals
Mature male mice (C57Bl/6; Seac Yoshitomi, Fukuoka, Japan) were housed in a light-, temperature- and humidity-controlled room (12:12-h light:dark cycle, lights on/off at 0700 and 1900 hours, respectively; 21±1 °C; 55±5% relative humidity). The mice were allowed free access to 60% high-fat (HF) food (item D12492: 20% protein, 20% carbohydrate and 60% fat, 5.2 kcal g−1; Research Diet, Tokyo, Japan) and control diet (item D12450B: 3.9 kcal g−1; Research Diet) and water. The HF food contained soybean oil (25/773.85 g) and lard (245/773.85 g). HF food was administered for 6 weeks, while the mice were between 8 and 14 weeks of age. All the animals were treated in accordance with the Oita University Guidelines for the Care and Use of Laboratory Animals.
Cilnidipine preparation and treatment
Cilnidipine (Mochida Pharmaceutical, Tokyo, Japan) and nicardipine (Sigma-Aldrich, Tokyo, Japan) were dissolved in vehicle (adjusted to pH 6.8–7.4). Each solution was prepared on the day that it was administered. We monitored drinking water of both the control and the cilnidipine treatment groups. Mice were selected and divided into two treatment groups. For the HF cilnidipine (HF-CIL) and HF nicardipine treatment groups, cilnidipine or nicardipine was added to the drinking water at a dose of 0.2 g l−1 for 14 consecutive days. The doses of cilnidipine and nicardipine were based on our preliminary data and a previous study.17 As mentioned above, the control HF treatment group received untreated water for 14 consecutive days (last 2 weeks). The cumulative food intake was measured once every 24 h for each of the 14 days of treatment. Body weight, the histology of epididymal WAT, and the adipocytokine mRNA expression levels were examined and measured in all the animals at the end of the 14-day treatment period. WAT was dissected from the epididymal fat, mesenteric fat and retroperitoneal fat as visceral fat. The body fat mass was measured to assess changes in body fat accumulation in mice. The tissues were removed, weighed and immediately frozen in liquid nitrogen and then stored at −80 °C until subjected to mRNA extraction. The blood was withdrawn from the jugular vein, and the serum was separated and immediately frozen at −20 °C until assayed. The serum glucose, insulin, triglyceride and free fatty-acid levels were measured using commercially available assay kits (Wako Chemical, Tokyo, Japan).
Measurement of blood pressures
After treatment for 2 weeks, the systolic and diastolic blood pressures (SBP and DBP) and heart rate (HR) of the offspring were measured noninvasively using the tail-cuff method in conscious mice, as described previously (BP-2000; Visitech-Systems, Apex, NC, USA).18
Histological analysis
Epididymal WAT samples were fixed with 10% formalin and embedded in paraffin. Sections of 20 μm were cut and stained with hematoxylin and eosin to examine the histology of the white adipocytes used in the microscopy analysis system (Olympus, Tokyo, Japan).
Measurement of triglycerides in tissues
A total of 100 mg of epididymal WAT was homogenized using a polytron homogenizer (NS-310E; Micro Tech Nichion, Chiba, Japan) for 1 min in 2 ml of a solution containing NaCl at 1.5 × 10−4 mol l−1 (150 mM), 0.1% Triton X-100 and Tris at 1 × 10−5 mol l−1 (10 mM). The triglyceride content of this solution was determined using a commercially available kit (Wako Chemical).
Intraperitoneal glucose and ITT
After 4-h fasting, for the intraperitoneal glucose tolerance test or the ITT, the mice were treated with 2 g of glucose per kg body weight or 0.5 mU of regular insulin per g body weight via an intraperitoneal injection (Nobolin R; Novo Nordisk, Bagsvaerd, Denmark). Blood was sampled from the tail vein before and 30, 60 and 90 min after these injections, and the serum glucose and insulin concentrations were determined.
Cell culture and differentiation
Murine 3T3-L1 preadipocytes were obtained from the American Type Culture Collection (Dainippon Pharmaceutical, Osaka, Japan). Dulbecco’s Modified Eagle Medium, fetal bovine serum, penicillin, streptomycin, D-biotin, insulin, dexamethasone and all other cell culture reagents were obtained from Wako Chemical. Cells were grown at 37 °C in 5% CO2 in Dulbecco’s Modified Eagle Medium containing 25 mM HEPES, 8 mg l−1 D-biotin, 100 U ml−1 penicillin and 10% fetal bovine serum (conditioned medium) and were seeded in six-well plates when used for experiments. Differentiation into mature adipocytes was induced by exposing the cells to conditioned medium supplemented with 1 μm of dexamethasone and 10 μg ml−1 of insulin (induction medium) for 2 days. Cells were then incubated with medium containing 5 μg ml−1 of insulin. After 2 days, the medium was replaced with the conditioned medium. Subsequently, the conditioned medium was changed regularly every 2 days. We have examined the effects of cilnidipine on adiponectin mRNA expression and secretion of adiponectin in 3T3-L1 adipocyte 12 h after CIL treatment. 3T3-L1 cells were pretreated with the concentration of cilnidipine (200 μm) for 12 h.
Real-time quantitative reverse transcription PCR
mRNAs were amplified using PCR and quantified using real-time quantitative PCR as follows. The total cellular RNA was prepared from selected mouse tissues using TRIzol (Lifetech, Tokyo, Japan) according to the manufacturer’s protocol. Total RNA (20 μg) was electrophoresed on 1.2% formaldehyde-agarose gels. The RNA quality and quantity were assessed using ethidium bromide agarose gel electrophoresis and by calculating the 260:280 nm absorbance ratios. cDNA was synthesized from 150 ng of total RNA in a volume of 20 μl using a ReverTra-Dash reverse transcriptase kit (Toyobo, Tokyo, Japan) with random hexamer primers. Reactions were diluted to 50 μl with sterile distilled H2O and stored at −20 °C. Primers were provided as qpre-optimized kits from Assay-on-Demand: resistin, IL-6 (the primers were generated by Nihon Gene Research Lab, Sendai, Japan), TNF-alpha (Mm00443258ml) and adiponectin (Mm00456425m1). Primers for ribosomal RNA, used as an internal control, were also provided as a pre-optimized kit (Hs99999901). PCR amplification was performed using an ABI PRISM 7000 sequence detector (Applied Biosystems, Foster City, CA, USA) in 50-μl volumes containing 100 ng of cDNA template in a PCR Master Mix (Roche, Nutley, NJ, USA) according to the following program: 50 °C for 2 min, 95 °C for 10 min, 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Samples were analyzed in duplicate. The results were analyzed using Sequence Detection Software (Applied Biosystems), and the level of mRNA expression was normalized to that of ribosomal RNA as outlined in the Perkin-Elmer User Bulletin No. 2 (Perkin-Elmer, Wellesley, MA, USA).
Determination of levels of serum resistin, IL-6, TNF-alpha, and total and high-molecular weight adiponectin
The levels of WAT, serum resistin, IL-6, TNF-alpha, and total and HMW adiponectin were determined using an enzyme-linked immunosorbent assay kit (BioSource, Tokyo, Japan; Otsuka Pharmaceutical, Tokushima, Japan) and an optical density reader according to the manufacturer’s instructions.
Statistical analysis
All data are expressed as the mean±s.d. An unpaired t-test or analysis of variance post-hoc Bonferroni test was used to analyze statistical differences using StatView 4.0 (SAS Institute, Cary, NC, USA)
Results
Effects of cilnidipine treatment on body weight, adiposity, glucose and insulin
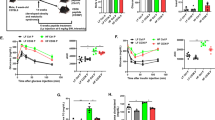
Figure 1a summarizes the body weight and Figures 1b and c show the average food and drink intakes over the 6 weeks of the study period. No significant differences in the daily HF food consumption and body weight were noted between the cilnidipine-treated (HF-CIL) and the non-treated (HF) animals. Cilnidipine treatment, when compared with the results in the non-treated HF group, did not affect adiposity as assessed using the epididymal WAT weight (HF, 1.5±0.2 g vs. HF-CIL, 1.6±0.2 g, P>0.1) or the mesenteric, retroperitoneal WAT weight and the triglyceride content of the epididymal WAT (Figures 1d–f, P>0.1). We did not observe any notable morphological differences between the two groups (data not shown). In addition, both the serum glucose and insulin levels were not decreased in the HF-CIL group, compared with the untreated HF group (Figures 2a and b, P>0.1). The serum triglyceride and free fatty-acid levels were not significantly changed in the HF-CIL group (Figures 2c and d, P>0.1).
Effects of cilnidipine on (a) body weight changes (b) daily average food intake, (c) daily average drink intake, (d) weights of epididymal, (e) mesenteric and (f) retroperitoneal WAT and (g) triglyceride content of epididymal WAT in DIO mice. Results are shown for the control group not treated with cilnidipine (HF) and for the experimental group treated with cilnidipine (HF-CIL). Each value and vertical bar represents the mean±s.d. (n=4 for each parameter).
Effects of cilnidipine on (a) serum glucose, (b) serum insulin, (c) serum triglycerides, (d) serum free fatty acids and (e, f) glucose level changes during the intraperitoneal glucose and ITT in DIO mice. Results are shown for the HF and HF-CIL groups. Each value and vertical bar represents the mean ±s.d. (*P<0.05, **P<0.01 vs. control, n=4 for each parameter).
Effects of cilnidipine treatment on blood pressure and HR
The SBP and DBP were both higher in the DIO group, compared with the control groups (P<0.01). The SBP and DBP were not significantly changed before cilnidipine treatment (SBP: HF, 121±4 mm Hg vs. HF-CIL, 122±5 mm Hg; DBP: HF, 98±3 mm Hg vs. HF-CIL, 99±4 mm Hg, P>0.1 for each parameter). As expected, the SBP and DBP decreased with cilnidipine treatment (SBP: HF, 115±3 mm Hg vs. HF-CIL, 98±2 mm Hg; DBP: HF, 94±3 mm Hg vs. HF-CIL, 84±2 mm Hg, P<0.01 for each parameter). The HR tended to decrease, but the change was not significant compared with the controls (pre HR: HF, 605±52 vs. HF-CIL, 627±57; post HR: HF, 578±65 vs. HF-CIL, 593±72, P>0.1).
Intraperitoneal glucose and ITT
During the intraperitoneal glucose tolerance test, the CIL-treated mice showed lowered levels of blood glucose at each measurement point, compared with the controls (Figure 2e; P<0.01 for each parameter). The lowered glucose level in the CIL-treated mice persisted even 90 min after the initiation of the test. The levels of circulating insulin during the test were not significantly changed by CIL treatment. During the intraperitoneal ITT, the CIL-treated mice showed lowered levels of blood glucose at each measurement point, compared with the controls (Figure 2f; P<0.05 or <0.01 for each parameter).
Effects of nicardipine treatment on food intake body weight, glucose tolerance test and adiponectin levels
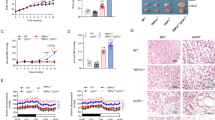
No significant differences in the daily HF food consumption and body weight were observed between the nicardipine-treated and the non-treated animals (Figures 3a and b). The SBP and DBP decreased significantly with nicardipine treatment (SBP: HF, 120±3 mm Hg vs. HF nicardipine, 104±2 mm Hg; DBP: HF, 96±3 mm Hg vs. HF nicardipine, 87±1 mm Hg, P<0.01 for each parameter). The improvements of glucose metabolism during the intraperitoneal glucose tolerance test were not observed in mice treated with nicardipine (Figure 3c). In addition, the levels of HMW and total adiponectin in the sera were not significantly changed by nicardipine treatment (Figure 3d).
Effects of nicardipine on (a) body weight changes (b) daily average food intake, (c) glucose levels during the intraperitoneal glucose tolerance test and (d) serum total and HMW adiponectin levels in DIO mice. Results are shown for the control group not treated with nicardipine (HF) and for the experimental group treated with nicardipine (HF nicardipine). Each value and vertical bar represents the mean±s.d. (n=4 for each parameter).
Effects of cilnidipine treatment on inflammatory adipocytokine levels
We examined the mRNA expression and serum levels of TNF-alpha, resistin and IL-6 in epididymal WAT and found that none of these levels (Figures 4a–c) were altered in the HF-CIL group, compared with the HF group (TNF-alpha mRNA: HF, 100±14% vs. HF-CIL, 98±25%; resistin mRNA: HF, 100±20% vs. HF-CIL, 113±32%; IL-6 mRNA: HF, 100±14% vs. HF-CIL, 88±23%, P>0.1 for each parameter).
Effects of cilnidipine on (a) TNF-alpha mRNA, IL-6 mRNA and resistin mRNA expression levels in epididymal WAT, and (b) circulating TNF-alpha, IL-6, (c) circulating resistin, (d) adiponectin mRNA expression levels in WAT, (e) serum adiponectin levels and (f) the ratio of serum HMW/total adiponectin in DIO mice. Results are shown for the HF-CIL and HF groups. Each value and vertical bar represents the mean ±s.d. (*P<0.05, **P<0.01 vs. control, n=4 for each parameter).
Effect of cilnidipine treatment on adiponectin level
Figure 4d shows that the adiponectin mRNA levels in epididymal and retroperitoneal WAT were increased in the HF-CIL group, compared with the HF group (epididymal WAT: HF, 100±16% vs. HF-CIL, 178±26%, P<0.05; mesenteric WAT: HF, 100±12% vs. HF-CIL, 111±15%, P>0.1; retroperitoneal WAT: HF, 100±16% vs. HF-CIL, 162±34%, P<0.05). Similarly, Figure 4e shows that the serum levels of total adiponectin and HMW adiponectin were both significantly increased in the HF-CIL group, compared with the control HF group (P<0.05 or <0.01). Of note, the ratio of HMW/total adiponectin in the sera increased dramatically with CIL treatment (Figure 4f).
Effect of cilnidipine treatment on total and HMW adiponectin levels in adipocytes
Figures 5a and b show the levels of adiponectin in 3T3-L1 cells after cilnidipine treatment. The mRNA expression level of adiponectin in the 3T3-L1 cells was increased in the CIL group, compared with the CONT group (P<0.05). Similarly, the level of adiponectin in the medium was increased in the 3T3-L1 cells (CONT, 2.4±0.2 μg ml−1 vs. CIL, 3.5±0.2 μg ml−1, P<0.05). There was no significantly change in the levels of TNF-alpha and INF-gamma between the groups (TNF-alpha: CONT, 4.1±1.3 pg ml−1 vs. CIL, 5.1±0.9 pg ml−1, P>0.1; INF-gamma: CONT, 2.3±0.5 pg ml−1 vs. CIL, 2.6±0.4 pg ml−1, P>0.1).
Effects of cilnidipine on (a) adiponectin mRNA expression and (b) secretion of adiponectin in 3T3-L1 adipocyte 12 h after CIL treatment at a dose of 200 μm. Results are shown for the HF-CIL and HF groups. Each value and vertical bar represents the mean ±s.d. (*P<0.05 vs. control, n=4 for each parameter).
Discussion
In the present study, we have demonstrated that the calcium channel inhibitor cilnidipine improves glucose and insulin tolerance accompanied by an increase in adiponectin in DIO mice. Obesity and/or fat accumulation are well known to lead potentially to insulin resistance, although a reduction in body weight or adiposity can minimize problems, such as insulin resistance, that are often associated with obesity-related metabolic disorders.19 In the present study, however, cilnidipine treatment did not affect either the body weight or adiposity. Therefore, cilnidipine treatment may modify glucose metabolism and/or insulin sensitivity independent of body adiposity.
The pharmacological properties of cilnidipine, which acts as an L-type and an N-type calcium channel antagonist, should be mentioned.7, 20 L-type and/or N-type calcium channel drugs are known to affect insulin resistance favorably,7, 20 and the regulation of sympathetic nerves may affect insulin signaling, thereby affecting the activity of intracellular signaling molecules including adipocytes.21, 22, 23 In addition to signaling molecules in adipocytes, a number of factors have been shown to be involved in regulating insulin resistance by adipocytokines, such as adiponectin and TNF-alpha.8, 9, 10, 11 In the present study, we focused on the effect of cilnidipine on the adipocytokines resistin, IL-6, TNF-alpha and adiponectin. Treatment with cilnidipine did not affect the levels of resistin, IL-6 or TNF-alpha in serum or WAT. However, we found that cilnidipine treatment regulated the levels of adiponectin in WAT and sera. Adiponectin is known to be an insulin-sensitizing adipocytokine24 that regulates glucose metabolism by accelerating insulin signaling and glucose uptake in the liver and skeletal muscles.25 Adiponectin also regulates markers of inflammation, contributing to its positive effect on insulin sensitivity.26, 27 These findings suggested that adiponectin might be involved in cilnidipine-induced improvements in insulin sensitivity in DIO mice. In the present study, L-type calcium channel antagonist nicardipine did not significantly influence the glucose metabolism and adiponectin levels in DIO mice. Although the detailed mechanisms remain unknown, N-type calcium channels may effectively influence adiponectin and/or cilnidipine may have pleiotropic effects on adiponectin.
Adiponectin is generally present in serum as a trimer, hexamer or HMW form.27 Among the forms of adiponectin, the HMW adiponectin form is the most active and confers a protective effect on blood vessels.28 Furthermore, HMW adiponectin appears to be better associated with insulin sensitivity than total adiponectin and to be strongly associated with a lower risk for incident diabetes,29 coronary artery disease30 and cerebrovascular disease. Interestingly, cilnidipine treatment accelerated the secretion of HMW adiponectin. This result suggests that cilnidipine may improve glucose metabolism in a manner involving HMW adiponectin. The present study provides novel insights into the involvement of cilnidipine as a therapeutic tool in the treatment of metabolic syndrome, including hypertension and insulin resistance. Several studies presented the nifedipine influenced on glucose metabolism, lipid oxidation and adipocyte dysfunction.31, 32 Thus, it is possible that several calcium channel blockers, including cilnidipine, have an action on adipocyte.
The present study has several limitations. First, this study is pharmacologically experiment and the dose of cilnidipine in this study is relatively high. Second, the number of mice might not be enough. Third, the experiments using specific inhibitor of N-type Ca channel or deficient mice of the channel would be useful. Further basic and clinical studies are needed to clarify the detail mechanisms of cilnidipine.
In summary, the results of our study suggest that cilnidipine treatment may prevent the development of insulin resistance without affecting body adiposity in DIO mice. Associated changes in the levels and/or activity of adiponectin, especially HMW adiponectin, may contribute to the beneficial therapeutic effect of cilnidipine on metabolic disorders.
References
Kong AP, Chan NN, Chan JC . The role of adipocytokines and neurohormonal dysregulation in metabolic syndrome. Curr Diabetes Rev 2006; 2: 397–407.
Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ . The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010; 56: 1113–1132.
Pershadsingh HA, Lee LY, Snowdowne KW . Evidence for a sodium/calcium exchanger and voltage-dependent calcium channels in adipocytes. FEBS Lett 1989; 244: 89–92.
Nanberg E, Connolly E, Nedergaard J . Presence of a Ca2+-dependent K+ channel in brown adipocytes. Possible role in maintenance of alpha 1-adrenergic stimulation. Biochim Biophys Acta 1985; 844: 42–49.
Araki K, Masaki T, Katsuragi I, Tanaka K, Kakuma T, Yoshimatsu H . Telmisartan prevents obesity and increases the expression of uncoupling protein 1 in diet-induced obese mice. Hypertension 2006; 48: 51–57.
Sugimoto K, Qi NR, Kazdova L, Pravenec M, Ogihara T, Kurtz TW . Telmisartan but not valsartan increases caloric expenditure and protects against weight gain and hepatic steatosis. Hypertension 2006; 47: 1003–1009.
Yagi S, Goto S, Yamamoto T, Kurihara S, Katayama S . Effect of cilnidipine on insulin sensitivity in patients with essential hypertension. Hypertens Res 2003; 26: 383–387.
Matsuzawa Y . The metabolic syndrome and adipocytokines. FEBS Lett 2006; 580: 2917–2921.
Kadowaki T, Yamauchi T . Adiponectin and adiponectin receptors. Endocr Rev 2005; 26: 439–451.
Dandona P, Aljada A, Bandyopadhyay A . Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 2004; 25: 4–7.
Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS . Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997; 389: 1997.
Galic S, Oakhill JS, Steinberg GR . Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010; 316: 129–139.
Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H . Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology 2004; 40: 177–184.
Masaki T, Chiba S, Yasuda T, Tsubone T, Kakuma T, Shimomura I, Funahashi T, Matsuzawa Y, Yoshimatsu H . Peripheral, but not central, administration of adiponectin reduces visceral adiposity and upregulates the expression of uncoupling protein in agouti yellow (Ay/a) obese mice. Diabetes 2003; 52: 2266–2273.
Shimada K, Miyazaki T, Daida H . Adiponectin and atherosclerotic disease. Clin Chem Acta 2004; 344: 1–12.
Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, Matsuzawa Y . Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 2001; 50: 1126–1233.
Fan YY, Kohno M, Nakano D, Ohsaki H, Kobori H, Suwarni D, Ohashi N, Hitomi H, Asanuma K, Noma T, Tomino Y, Fujita T, Nishiyama A . Cilnidipine suppresses podocyte injury and proteinuria in metabolic syndrome rats: possible involvement of N-type calcium channel in podocyte. J Hypertens 2010; 28: 1034–1043.
Yono M, Yamamoto Y, Yoshida M, Ueda S, Latifpour J . Effects of doxazosin on blood flow and mRNA expression of nitric oxide synthase in the spontaneously hypertensive rat genitourinary tract. Life Sci 2007; 8: 218–222.
Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W, Maerker E, Matthaei S, Schick F, Claussen CD, Haring HU . Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes 1999; 48: 1113–1119.
Masuda T, Ogura MN, Moriya T, Takahira N, Matsumoto T, Kutsuna T, Hara M, Aiba N, Noda C, Izumi T . Beneficial effects of L- and N-type calcium channel blocker on glucose and lipid metabolism and renal function in patients with hypertension and type ii diabetes mellitus. Cardiovasc Ther 2011; 29: 46–53.
Thorens B . Glucose sensing and the pathogenesis of obesity and type 2 diabetes. Int J Obes (Lond) 2008; 32: S62–S71.
McCarty MF . Elevated sympathetic activity may promote insulin resistance syndrome by activating alpha-1 adrenergic receptors on adipocytes. Med Hypotheses 2004; 62: 830–838.
Mulder AH, Tack CJ, Olthaar AJ, Smits P, Sweep FC, Bosch RR . A drenergic receptor stimulation attenuates insulin-stimulated glucose uptake in 3T3-L1 adipocytes by inhibiting GLUT4 translocation. Am J Physiol Endocrinol Metab 2005; 289: E627–E633.
Stefan N, Vozarova B, Funahashi T, Matsuzawa Y, Weyer C, Lindsay RS, Youngren JF, Havel PJ, Pratley RE, Bogardus C, Tataranni PA . Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes 2002; 50: 1884–1888.
Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T . Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002; 8: 1288–1295.
Shetty S, Kusminski CM, Scherer PE . Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci 2009; 30: 234–239.
Hirose H, Yamamoto Y, Seino-Yoshihara Y, Kawabe H, Saito I . Serum high-molecular-weight adiponectin as a marker for the evaluation and care of subjects with metabolic syndrome and related disorders. J Atheroscler Thromb 2010; 17: 1201–1211.
Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y . Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res 2004; 94: e27–e31.
Nakashima R, Kamei N, Yamane K, Nakanishi S, Nakashima A, Kohno N . Decreased total and high molecular weight adiponectin are independent risk factors for the development of type 2 diabetes in Japanese-Americans. J Clin Endocrinol Metab 2006; 91: 3873–3877.
von Eynatten M, Humpert PM, Bluemm A, Lepper PM, Hamann A, Allolio B, Nawroth PP, Bierhaus A, Dugi KA . High-molecular weight adiponectinis independently associated with extent of coronary artery disease in men. Atherosclerosis 2008; 199: 123–128.
Iwai M, Kanno H, Inaba S, Senba I, Sone H, Nakaoka H, Nifedipine Horiuchi M . A calcium-channel blocker, attenuated glucose intolerance and white adipose tissue dysfunction in type 2 diabetic KK-A(y) mice. Am J Hypertens 2011; 24: 169–174.
Tian Z, Miyata K, Tabata M, Yano M, Tazume H, Aoi J, Takahashi O, Araki K, Kawasuji M, Oike Y . Nifedipine increases energy expenditure by increasing PGC-1α expression in skeletal muscle. Hypertens Res 2011; 34: 1221–1227.
Acknowledgements
This work was supported by a grant from the Japanese Ministry of Education, Science and Culture and Research on Measures for Intractable Diseases, Japanese Ministry of Health, Welfare and Labor, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ueno, D., Masaki, T., Gotoh, K. et al. Cilnidipine regulates glucose metabolism and levels of high-molecular adiponectin in diet-induced obese mice. Hypertens Res 36, 196–201 (2013). https://doi.org/10.1038/hr.2012.141
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2012.141
Keywords
This article is cited by
-
T lymphocyte depletion ameliorates age-related metabolic impairments in mice
GeroScience (2021)
-
Effects of antihypertensive drugs losartan and levamlodipine besylate on insulin resistance in patients with essential hypertension combined with isolated impaired fasting glucose
Hypertension Research (2016)
-
Serum soluble (pro)renin receptor levels in patients with essential hypertension
Hypertension Research (2014)