Abstract
The objective of this study is to examine the effects of thiazide diuretics, plus medium-dose losartan versus maximal-dose angiotensin II receptor blockers (ARBs) on blood pressure (BP) in Japanese patients with uncontrolled hypertension despite the use of medium-dose ARBs. Hypertensive patients in whom BP was inadequately controlled by treatment with medium-dose ARBs alone or with calcium-channel blockers were enrolled. Patients were randomly assigned to a fixed-dose combination of 50 mg per day losartan and 12.5 mg per day hydrochlorothiazide (HCTZ; n=98), or to a maximal dose of current ARBs (n=95). The reduction in office BP from baseline was significantly larger in the losartan/HCTZ group than in the maximal-dose ARB group (systolic BP −22.7±13.7 vs. −11.7±13.0 mm Hg, diastolic BP −9.6±10.9 vs. −4.5±11.0 mm Hg; P<0.01, respectively). The proportion of patients in whom the therapeutic target BP was achieved was greater in the losartan/HCTZ group than in the maximal-dose ARB group (59.2 vs. 26.3%; P<0.001). Both early-morning and evening BP were controlled more effectively over 1 year of treatment in the losartan/HCTZ group than in the maximal-dose ARB group (the mean BP difference between the groups, early-morning: 5.6 mm Hg (P=0.001), evening: 3.8 mm Hg (P=0.049)). Adverse changes in serum potassium and uric acid were observed in the losartan/HCTZ group; however, both changes were very slight, and the values were still within the normal range. The concomitant usage of losartan and HCTZ had no influence on glucose metabolism and lipid profiles. Declines in plasma N-terminal pro-brain natriuretic peptide levels and urinary albumin excretion were observed in the losartan/HCTZ group, but not in the maximal-dose ARB group. Switching from medium-dose ARBs to losartan plus HCTZ reduced both office and home BP efficiently in patients with uncontrolled hypertension.
Similar content being viewed by others
Introduction
Although it is well established that hypertension causes organ damage and increases the risk of adverse cardiovascular and renal outcomes,1, 2 many hypertensive patients in Japan have not received the medical treatment necessary to achieve the recommended target blood pressure (BP).3 Among various antihypertensive drugs, angiotensin II receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEIs) are considered to have cardioprotective effects beyond their BP-reducing effects.4, 5 In particular, ARBs have become widely used in Japan, because they do not produce a dry cough (unlike ACEIs) and are therefore well tolerated. However, it is often difficult to obtain sufficient BP-lowering effects with the usual dose of ARBs administered alone, because hypertensive patients with a low renin status and/or a high salt intake may not respond well to these drugs. In such cases, another strategy is required: either an increase in the dose of the current ARB or the addition of another antihypertensive drug. Concomitant use of thiazide diuretics with ARBs is recommended by various guidelines, including those published by the Japanese Society of Hypertension (JSH),1, 2 because thiazide diuretics have a complementary mechanism of action that involves the induction of renal sodium excretion.2 However, the rate of diuretic prescription in Japan is still low because of the associated adverse metabolic effects, including hypokalemia, hyperuricemia, and the harmful influences on glucose and lipid metabolism.6 Furthermore, it is still controversial whether a combination of rennin-angiotensin system (RAS) inhibitors and diuretics confers beneficial effects with regard to renal7, 8 and cardiovascular outcomes.9 However, complete blockade of the RAS through increased doses of ARBs is recommended, based on the clinical benefits observed in patients with cardiovascular disease.10
In Japan, a single tablet containing losartan (50 mg) and hydrochlorothiazide (HCTZ, 12.5 mg; Preminent; MSD K.K., Tokyo, Japan) has been approved for clinical use. ARBs are thought to have favorable effects on glucose metabolism,11 and to increase the levels of serum potassium via the anti-aldosterone effect. Furthermore, the ARB losartan is known to have a uricosuric effect via the inhibition of urate transporter-1.12 Therefore, losartan plus HCTZ could be a potent therapeutic option, especially for Japanese patients with uncontrolled hypertension, because in addition to habitual high dietary salt intake,13 the frequency of an allele related to salt sensitivity is thought to be high in the Japanese population compared with Caucasians.14
The Kobe combination therapy of losartan plus HCTZ (losartan/HCTZ), effect and safety trial (Kobe-CONNECT) Study was designed to compare the effectiveness and safety of losartan/HCTZ with that of the maximal dose of the currently prescribed ARBs in Japanese patients whose BPs were uncontrolled, despite treatment with a medium dose of ARBs alone or with calcium-channel blockers (CCBs).
Methods
Study population
The study participants, aged over 30 years, were recruited from 24 participating centers in and around Kobe, Japan (see Appendix). The entry period was from September 2008 to December 2009. We enrolled consecutive hypertensive outpatients who had not achieved therapeutic target levels of office BP, as defined by the 2004 JSH guidelines (130/85 mm Hg for patients aged less than 64 years, 140/90 mm Hg for patients aged 65 years or more, and 130/80 mm Hg for patients with diabetes mellitus and/or chronic kidney disease) following treatment with moderate doses of ARBs alone or with CCBs for at least 8 weeks. Patients with severe uncontrolled hypertension (diastolic BP ⩾110 mm Hg), secondary hypertension, white coat hypertension, hyperuricemia with a past history of gout attack, poorly controlled (glycated hemoglobin A1c ⩾8.0%) or insulin-treated diabetes mellitus, cardiovascular events (including myocardial infarction and stroke) within the preceding 6 months, severe liver dysfunction, or renal failure (serum creatinine ⩾2.0 mg dl−1) were excluded from the study. Patients treated with any type of diuretics were also excluded. All subjects provided written informed consent before enrolment. The study was approved by the Ethics Committee of Kobe University Graduate School of Medicine and registered at UMIN-CTR with identification number 000001389.
Study protocol
The Kobe-CONNECT Study was a prospective, randomized, open-label, comparative multicenter study. Eligible patients were randomly assigned to switch to a fixed-dose combination therapy with 50 mg per day losartan (changed from current ARB) plus 12.5 mg per day HCTZ, or to a maximal-dose of the currently prescribed ARB approved by the Ministry of Health, Labour and Welfare in Japan (losartan: usual dose=50 mg per day, maximal dose=100 mg per day; valsartan: usual dose=80 mg per day, maximal dose=160 mg per day; candesartan: usual-dose=8 mg per day, maximal dose=12 mg per day; olmesartan: usual dose=20 mg per day, maximal dose=40 mg per day; telmisartan: usual dose=40 mg per day, maximal dose=80 mg per day; or irbesartan: usual dose=100 mg per day, maximal dose=200 mg per day). For the randomization, the physician who enrolled the patients made a phone call to a central randomization center, and one of two treatment arms was automatically assigned. Subjects taking CCBs before randomization continued on the same CCBs. No other antihypertensive drugs or dose titration was permitted for 12 weeks. If the patients did not reach the BP target at 12 weeks, CCBs could be added. The primary outcome was the change in office BP from the baseline value at 12 weeks. The secondary endpoints included the change in home BP and laboratory tests. To investigate longer-term safety and efficacy, we continued the study until 48 weeks. Clinic BPs were measured using the auscultation method using a mercury sphygmomanometer after 5 min of rest in a seated position at 0, 12 and 48 weeks. Home BPs were measured at 0, 4, 8, 12, 24, 36 and 48 weeks using an automatic digital sphygmomanometer (HEM-7051-HP, Omron Healthcare, Kyoto, Japan). The self-monitoring of morning BP was made within 1 h of waking, before the patient had taken any antihypertensive drugs. Evening BP was measured at any given time between supper and bedtime. The mean pressure was calculated as diastolic BP plus one-third of pulse pressure. Throughout the study, lipid-lowering, anti-diabetic, or uricosuric drug regimens were continued without change in dose or usage.
Laboratory tests
Blood tests, including electrolytes, lipid profile, uric acid (UA), hemoglobin A1c, creatinine, N-terminal pro-brain natriuretic peptide (NT-proBNP) and highly sensitive C-reactive protein were conducted at baseline and 48 weeks. The urine albumin-to-creatinine ratio was also estimated using casual urine samples at the same time. The estimated glomerular filtration rate (eGFR) was calculated using the following formula of the Japanese Society of Nephrology: eGFR (ml min−1 per 1.73 m2)=194 × (serum creatinine)−1.094 × age−0.287 ( × 0.739 if female).15 Chronic kidney disease was defined as eGFR <60 ml min−1 per 1.73 m2 and/or the presence of proteinuria.
Study power calculation
We estimated that the decrease in office systolic BP in the losartan/HCTZ group and the maximal-dose ARB group would be 17±10 and 13±10 mm Hg, respectively, based on the information in a previous report.16 Under the statistical conditions of α=0.05 and 1−β=0.8, a total of 200 patients (100 patients per group) were necessary to detect a significant difference between the groups.
Statistical analysis
All data are expressed as the mean±s.d., unless otherwise specified. Between-treatment differences were investigated using an unpaired t-test or the Mann–Whitney test according to the data distribution, with or without normality. The χ2-test or Fisher’s exact test was used to compare categorical values between the groups. As the changes in office and home BP during the follow-up period were repeatedly measured and autocorrelation was assumed, the changes were evaluated using a generalized estimating equation with a first-order autocorrelation structure.17, 18 A P-value <0.05 was the criterion for statistical significance. All analyses were performed using Stata/IC 10.1 for Windows (StataCorp LP, College Station, TX, USA).
Results
Patient characteristics
A total of 200 hypertensive patients who were eligible for this study were randomly assigned to the losartan/HCTZ group (n=100) or the maximal-dose ARB group (n=100). Seven patients were lost to follow-up because of patient requests. Evaluable BP data were available at the primary endpoint for 193 patients (98 and 95 patients, respectively). Seventy-five patients in the losartan/HCTZ group and 69 patients in the maximal-dose ARB group were followed up for 48 weeks. There were no significant differences in baseline clinical characteristics between the two randomized groups (Table 1). No difference in the distribution of pre-prescribed ARBs was observed between groups (Table 1). At baseline, 59.2% (58/98) of the losartan/HCTZ group and 54.7% (52/95) of the maximal-dose ARB group had been treated by combination therapy with CCBs (Table 1).
Changes in BP after 12 weeks of treatment
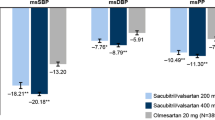
A within-treatment group analysis demonstrated that the seated office systolic and diastolic BP after 12 weeks of treatment were significantly reduced from baseline in both groups (losartan/HCTZ group; 153.7±11.1/84.0±11.1 to 131.1±14.3/74.4±9.3 mm Hg, respectively, P<0.01; maximal-dose ARBs; 154.6±11.8/85.4±11.2 to 142.9±14.1/80.9±11.9 mm Hg, respectively, P<0.01), whereas the reductions in both systolic and diastolic BP were significantly larger in the losartan/HCTZ group than in the maximal-dose ARB group (Figure 1). Changes in pulse and mean pressure from baseline were also larger in patients treated with losartan/HCTZ than in those treated with maximal-dose ARBs (Figure 1). At the 12-week visit, 58/98 (59.2%) of the patients in the losartan/HCTZ group and 25/95 (26.3%) in the maximal-dose ARB group had reached the therapeutic target BP values (P<0.001 for both). Regardless of the concomitant use of CCBs, similar reductions in BP were observed, except for the changes in diastolic BP in those taking CCBs (Figure 1).
Mean changes in the office BP from the baseline value to the value at 12 weeks in all participants (Total) and in participants treated without (−) or with (+) a regular dose of calcium channel blockers (CCBs) at baseline. Mean±s.d., *P<0.01 between treatments. SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; MP, mean pressure.
Efficacy and safety of losartan/HCTZ for 48 weeks
We continued the study to evaluate the 1-year safety and efficacy of the concomitant usage of losartan and HCTZ. After the primary endpoint, despite the addition of CCBs, one patient from the losartan/HCTZ group and six patients from the maximal-dose ARB group dropped out because of inadequate BP-lowering effects. However, the addition of CCBs enabled six patients from the losartan/HCTZ group and four patients from the maximal-dose ARB group to adhere to the follow-up schedule. In contrast, three patients stopped losartan/HCTZ treatment owing to excessive hypotension. In the losartan/HCTZ group, one patient experienced photosensitivity, and two patients complained of skin rashes. One patient in the maximal-dose ARB group discontinued the treatment because of hyperkalemia. Sixteen patients in the losartan/HCTZ group and 19 patients in the maximal-dose ARB group could not be followed up after the primary endpoint, because they withdrew from the study or moved far away.
Figure 2 shows the office systolic and diastolic BP at 0, 12 and 48 weeks in patients who completed the 1-year observation period. At 48 weeks, in accordance with the intensive BP lowering achieved using CCBs and the drop-out of some uncontrolled patients, the diastolic BP of the maximal-dose ARB group declined to almost the same level as that of the losartan/HCTZ group (76.9±10.1 vs. 75.6±9.1 mm Hg; P=0.4028). In contrast, the systolic BP was still significantly lower in the losartan/HCTZ group than in the maximal-dose ARB group (131.3±11.8 vs. 140.1±13.1 mm Hg; P<0.001). Thus, office systolic BP was controlled efficiently in the losartan/HCTZ group compared with the maximal-dose ARB group throughout the study (Figure 2; the mean difference was 6.4 mm Hg; P<0.001). At the 48-week visit, the percentage of patients who achieved their target BP improved in the maximal-dose ARB group, but remained lower than that in the losartan/HCTZ group (33.3 vs. 57.3%; P<0.01).
Home BP was also controlled more effectively in the losartan/HCTZ group compared with the maximal-dose ARB group until the end of follow-up (Figure 3). After controlling for autoregression, the mean systolic BP of the losartan/HCTZ group was 5.6 mm Hg lower during the early morning and 3.8 mm Hg lower in the evening than that in the maximal-dose ARB group (Figure 3, P=0.001 and 0.049, respectively).
Changes in laboratory parameters
Table 2 shows the summary of changes in laboratory parameters at the end of follow-up. Serum potassium levels decreased statistically in the losartan/HCTZ group, but not in the maximal-dose ARB group (Table 2). Serum UA levels also increased slightly but significantly in the losartan/HCTZ group (5.6±1.6 to 5.8±1.6 mg dl−1; P<0.05). However, there were no significant differences in changes in UA levels between the groups (Table 2). Despite the changes in serum potassium and UA, the values remained in the normal range, and none of the participants discontinued the losartan/HCTZ treatment as a result of hypokalemia or hyperuricemia. Exploratory analysis of the subgroups revealed that losartan/HCTZ increased UA levels only in patients with relatively low levels of UA (UA <7.0 mg dl−1; 5.0±1.3 to 5.4±1.5 mg dl−1; P<0.01, n=56), but not in those with high levels of UA at baseline (UA⩾ 7.0 mg dl−1; 7.6±0.7 to 7.4±1.0 mg dl−1; P=0.3814, n=15). No significant changes were found in the hemoglobin A1c levels after 48 weeks of treatment with either losartan/HCTZ or maximal-dose ARBs (Table 2). Neither group displayed a remarkable change in lipid profile (Table 2).
NT-proBNP levels were reduced significantly from baseline in the losartan/HCTZ group, but not in the maximal-ARB group (Table 2). Moreover, the urine albumin-to-creatinine ratio levels were decreased from baseline in patients treated with losartan/HCTZ compared with patients treated with maximal-dose ARBs (Table 2). However, the eGFR also declined modestly but significantly in the losartan/HCTZ group (Table 2). No significant changes in highly sensitive C-reactive protein levels were observed in either group (Table 2).
Discussion
A number of large clinical trials have provided evidence that RAS blockers protect against cardiovascular organ damage in addition to lowering BP.4, 5 However, as salt intake is still higher in Japan than in western countries,13 and salt supplementation reduce the efficacy of ARBs,19 hypertensive patients who are resistant to treatment with the usual dose of ARBs are not treated adequately. This study, the Kobe-CONNECT Study, demonstrated that switching to a fixed-dose combination of losartan and thiazide diuretics is superior to an increased dose of the current ARBs for strict BP management in these cases. Our findings may reflect the fact that a relatively high proportion of hypertensive patients in Asia are salt sensitive14 and respond to combination therapy with RAS blockers and diuretics better than Caucasians, as demonstrated in the Perindopril Protection Against Recurrent Stroke Study trial.20 Salt sensitivity is enhanced under various conditions, including diabetes, obesity, metabolic syndrome and chronic kidney disease.21, 22, 23 However, in the present study, multivariate analysis did not reveal a correlation between these factors and the response rate after the addition of diuretics (data not shown). As salt sensitivity has been reported to increase with age,24 the relatively advanced age of the participants in the present study (approximately 70 years old) may have affected the results.
Morning BP is also known as an independent risk factor for cardiovascular disease. Uzu et al.25 previously reported that excess salt intake induces the impairment of nocturnal BP reduction, and that thiazide diuretics shifted the circadian rhythm of BP from the non-dipper to the dipper type. The present study supports the notion that combination therapy with losartan/HCTZ is a more effective regimen for treating early-morning high BP than an increased dose of ARBs. In addition, losartan/HCTZ reduced pulse pressure more effectively than maximal-dose ARBs. Currently, pulse pressure is drawing attention not only as a surrogate marker of aortic stiffness, but also as a therapeutic target.26
Whereas the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) provided a trigger for reviewing the role of thiazide diuretics in antihypertensive therapy,27 physicians in Japan still tend to hesitate to use diuretics for fear of adverse metabolic effects.6 The ALLHAT Study demonstrated that chlorothalidone increased the risk of new-onset diabetes and the prevalence of hypokalemia, although low doses might retain the hypotensive activity with minimal side effects.27 In the present study, no remarkable changes in serum hemoglobin A1c levels were observed in patients treated with losartan/HCTZ, which implies that the concomitant use of losartan could neutralize the adverse effects associated with thiazide diuretics on glucose metabolism. Although the magnitude of the change was modest and within the normal range, serum potassium levels decreased significantly in the losartan/HCTZ group. Further research will be necessary to clarify whether the effect of extensive BP lowering with losartan/HCTZ may outweigh the risk of the adverse effects.
An increase in the serum UA was observed in the losartan/HCTZ group, but mainly in the subgroup with relatively low UA levels at baseline. Another study has recently reported that the concomitant use of losartan with diuretics reduced serum UA levels significantly in patients whose UA levels at baseline were over 7.0 mg dl−1.28 On the basis of these findings, we speculate that the increase in serum UA achieved with losartan/HCTZ treatment does not represent a serious issue from a clinical perspective.
It is controversial whether the combination of RAS inhibitors and diuretics confers beneficial effects with regard to renal outcome. The Gauging Albuminuria Reduction With Lotrel in Diabetic Patients With Hypertension trial has also demonstrated that the concomitant use of ACEIs and diuretics reduces microalbuminuria.7 However, secondary analysis of the Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension (ACCOMPLISH) trial revealed the progression of chronic kidney disease in patients treated with a combination of ACEIs/diuretics.8 In our study, the reduction in the urine albumin-to-creatinine ratio values was significantly larger in the losartan/HCTZ group than in the maximal-ARB group. However, as the eGFR also decreased in the losartan/HCTZ group, the concomitant usage of diuretics still requires careful attention to renal function. It has also been reported that treatment with a RAS inhibitor reduced urinary albumin excretion via the attenuation of oxidative stress and inflammation,29 but there were no changes in serum highly sensitive C-reactive protein levels in either group. It is possible, however, that pretreatment with medium-dose ARBs obscured the effect on highly sensitive C-reactive protein levels.
In our study, NT-proBNP levels at baseline were slightly above normal in both groups. Olsen et al.30 have reported that NT-proBNP levels predict cardiovascular events in patients with hypertension. The NT-proBNP levels significantly decreased in the losartan/HCTZ group, but not in the maximal-dose ARB group. Further research will be necessary to determine whether the reduction in NT-proBNP caused by the concomitant use of ARBs and diuretics influences cardiovascular events in hypertensive patients.
It is controversial which combination, ARBs plus diuretics or ARBs plus CCBs, is more desirable for strict BP management. The ACCOMPLISH trial revealed that the effect of the ACEI/CCB combination on cardiovascular outcomes was superior to that of ACEI/HCTZ.9 However, it should be noted that more than 50% of participants in this study did not achieve the target BP, despite receiving both ARBs and CCBs at baseline. Furthermore, intensive BP lowering using CCBs after the primary endpoint was still not enough for the maximal-dose ARB group (six patients dropped out) compared with the losartan/HCTZ group (only one patient dropped out), indicating that ARBs plus CCBs is not always sufficient for BP control in Japanese patients. There is no doubt that the strict management of hypertension is essential to prevent organ damage, and to reduce the risk of cardiovascular and renal events.1, 2 Previous studies have suggested that antihypertensive treatment is beneficial, and a BP target of <140 mm Hg is safely achievable even in elderly patients.31, 32 Furthermore, the 22-year follow-up of a randomized trial recently showed that older patients who were treated with diuretic-based therapy for isolated systolic hypertension survived significantly longer without a fatal cardiovascular event than placebo recipients.33 Therefore, the concomitant use of diuretics with ARBs provides an important option in the treatment of Japanese patients with uncontrolled hypertension.
In conclusion, a switch to medium-dose losartan/HCTZ treatment reduced both office and home BP more efficiently than an increased dose of ARBs in patients with uncontrolled hypertension, despite the use of medium-dose ARBs. Severe adverse effects were not noted in either group. The present findings provide novel and direct evidence to inform the selection of antihypertensive drugs for uncontrolled hypertension.
Study limitations
One of the limitations in the present study is the absence of a washout period before switching to fixed-dose losartan/HCTZ or maximal-dose ARBs. The increase in salt sensitivity induced by pretreatment with ARBs may explain the quicker onset of BP-lowering effects in the losartan/HCTZ group than in the maximal-dose ARB group, as shown in Figure 2. Another limitation is that changes in laboratory parameters could not be studied in all participants, although the enrollment for laboratory tests was not biased.
References
Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ito S, Iwao H, Kario K, Kawano Y, Kim-Mitsuyama S, Kimura G, Matsubara H, Matsuura H, Naruse M, Saito I, Shimada K, Shimamoto K, Suzuki H, Takishita S, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Ueshima H, Umemura S, Ishimitsu T, Rakugi H . The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009). Hypertens Res 2009; 32: 3–107.
Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A . ESH-ESC Task Force on the Management of Arterial Hypertension 2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension. J Hypertens 2007; 25: 1751–1762.
Ohkubo T, Obara T, Funahashi J, Kikuya M, Asayama K, Metoki H, Oikawa T, Takahashi H, Hashimoto J, Totsune K, Imai Y . Control of blood pressure as measured at home and office, and comparison with physicians’ assessment of control among treated hypertensive patients in Japan: First Report of the Japan Home versus Office Blood Pressure Measurement Evaluation (J-HOME) study. Hypertens Res 2004; 27: 755–763.
Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G . Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000; 342: 145–153.
Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H . Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359: 995–1003.
Mori H, Ukai H, Yamamoto H, Saitou S, Hirao K, Yamauchi M, Umemura S . Current status of antihypertensive prescription and associated blood pressure control in Japan. Hypertens Res 2006; 29: 143–151.
Bakris GL, Toto RD, McCullough PA, Rocha R, Purkayastha D, Davis P . Effects of different ACE inhibitor combinations on albuminuria: results of the GUARD study. Kidney Int 2008; 73: 1303–1309.
Bakris GL, Sarafidis PA, Weir MR, Dahlof B, Pitt B, Jamerson K, Velazquez EJ, Staikos-Byrne L, Kelly RY, Shi V, Chiang YT, Weber MA . Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet 2010; 375: 1173–1181.
Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ . Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008; 359: 2417–2428.
Peters S . Comparison of efficacy of low- (80 mg/day) and high- (160-320 mg/day) dose valsartan in the prevention of in-stent restenosis after implantation of bare-metal stents in type B2/C coronary artery lesions. Am J Cardiovasc Drugs 2008; 8: 83–87.
Mancia G, Grassi G, Zanchetti A . New-onset diabetes and antihypertensive drugs. J Hypertens 2006; 24: 3–10.
Hamada T, Ichida K, Hosoyamada M, Mizuta E, Yanagihara K, Sonoyama K, Sugihara S, Igawa O, Hosoya T, Ohtahara A, Shigamasa C, Yamamoto Y, Ninomiya H, Hisatome I . Uricosuric action of losartan via the inhibition of urate transporter 1 (URAT 1) in hypertensive patients. Am J Hypertens 2008; 21: 1157–1162.
Kawano Y, Ando K, Matsuura H, Tsuchihashi T, Fujita T, Ueshima H . Report of the Working Group for Dietary Salt Reduction of the Japanese Society of Hypertension: (1) Rationale for salt restriction and salt-restriction target level for the management of hypertension. Hypertens Res 2007; 30: 879–886.
Katsuya T, Ishikawa K, Sugimoto K, Rakugi H, Ogihara T . Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res 2003; 26: 521–525.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A . Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992.
Neutel JM, Kolloch RE, Plouin PF, Meinicke TW, Schumacher H . Telmisartan vs losartan plus hydrochlorothiazide in the treatment of mild-to-moderate essential hypertension--a randomised ABPM study. J Hum Hypertens 2003; 17: 569–575.
Liang K-Y . Estimating functions and approximate conditional likelihood. Biometrika 1987; 74: 695–702.
James C . QIC program and model selection in GEE analyses. Stata J 2007; 7: 209.
Ekinci EI, Thomas G, MacIsaac RJ, Johnson C, Houlihan C, Panagiotopoulos S, Premaratne E, Hao H, Finch S, O'Callaghan C, Jerums G . Salt supplementation blunts the blood pressure response to telmisartan with or without hydrochlorothiazide in hypertensive patients with type 2 diabetes. Diabetologia 2010; 53: 1295–1303.
Arima H, Anderson C, Omae T, Liu L, Tzourio C, Woodward M, Macmahon S, Neal B, Rodgers A, Chalmers J . Perindopril-based blood pressure lowering reduces major vascular events in Asian and Western participants with cerebrovascular disease: the PROGRESS trial. J Hypertens 2010; 28: 395–400.
Fuenmayor N, Moreira E, Cubeddu LX . Salt sensitivity is associated with insulin resistance in essential hypertension. Am J Hypertens 1998; 11: 397–402.
Chen J, Gu D, Huang J, Rao DC, Jaquish CE, Hixson JE, Chen CS, Chen J, Lu F, Hu D, Rice T, Kelly TN, Hamm LL, Whelton PK, He J . Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: a dietary intervention study. Lancet 2009; 373: 829–835.
Uzu T, Kimura G, Yamauchi A, Kanasaki M, Isshiki K, Araki S, Sugiomoto T, Nishio Y, Maegawa H, Koya D, Haneda M, Kashiwagi A . Enhanced sodium sensitivity and disturbed circadian rhythm of blood pressure in essential hypertension. J Hypertens 2006; 24: 1627–1632.
Zemel MB, Sowers JR . Salt sensitivity and systemic hypertension in the elderly. Am J Cardiol 1988; 61: 7H–12H.
Uzu T, Kimura G . Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation 1999; 100: 1635–1638.
McEniery CM, Spratt M, Munnery M, Yarnell J, Lowe GD, Rumley A, Gallacher J, Ben-Shlomo Y, Cockcroft JR, Wilkinson IB . An analysis of prospective risk factors for aortic stiffness in men: 20-year follow-up from the Caerphilly prospective study. Hypertension 2010; 56: 36–43.
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Jama 2002; 288: 2981–2997.
Kita T, Yokota N, Ichiki Y, Ayabe T, Etoh T, Tamaki N, Kato J, Eto T, Kitamura K . One-year effectiveness and safety of open-label losartan/hydrochlorothiazide combination therapy in Japanese patients with hypertension uncontrolled with ARBs or ACE inhibitors. Hypertens Res 2010; 33: 320–325.
Shishido T, Konta T, Nishiyama S, Miyashita T, Miyamoto T, Takasaki S, Nitobe J, Watanabe T, Takeishi Y, Kubota I . Suppressive effects of valsartan on microalbuminuria and CRP in patients with metabolic syndrome (Val-Mets). Clin Exp Hypertens 2011; 33: 117–123.
Olsen MH, Wachtell K, Tuxen C, Fossum E, Bang LE, Hall C, Ibsen H, Rokkedal J, Devereux RB, Hildebrandt P . N-terminal pro-brain natriuretic peptide predicts cardiovascular events in patients with hypertension and left ventricular hypertrophy: a LIFE study. J Hypertens 2004; 22: 1597–1604.
Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ . Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358: 1887–1898.
Ogihara T, Saruta T, Rakugi H, Matsuoka H, Shimamoto K, Shimada K, Imai Y, Kikuchi K, Ito S, Eto T, Kimura G, Imaizumi T, Takishita S, Ueshima H . Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56: 196–202.
Kostis JB, Cabrera J, Cheng JQ, Cosgrove NM, Deng Y, Pressel SL, Davis BR . Association between chlorthalidone treatment of systolic hypertension and long-term survival. JAMA 2011; 306: 2588–2593.
Acknowledgements
This study was supported by the Waksman Foundation of Japan, Inc.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This study was registered at UMIN-CTR with identification number 000001389.
Appendix
Appendix
Centers participating in the Kobe-CONNECT Study Group: Akashi Medical Center, Ako-City Hospital, Chibune General Hospital, Hyogo Brain and Heart Center, Hyogo Prefectural Awaji Hospital, Japanese Red Cross Kobe Hospital, Kasai City Hospital, Kobe Adventist Hospital, Kobe Century Memorial Hospital, National Hospital Organization Kobe Medical Center, Kobe Rosai Hospital, Kobe University Hospital, Konan Hospital, Miki City Hospital, Mitsubishi Kobe Hospital, Steel Memorial Hirohata Hospital, Osaka Saiseikai Nakatsu Hospital, Rokko Island Hospital, Sanda City Hospital, Shinko Hospital, Kakogawa East City Hospital, Takatsuki General Hospital, Toyooka Public Hospital, Yodogawa Christian Hospital.
Rights and permissions
About this article
Cite this article
Toh, R., Ishida, T., Nishimura, K. et al. Comparison of medium-dose losartan/hydrochlorothiazide and maximal-dose angiotensin II receptor blockers in the treatment of Japanese patients with uncontrolled hypertension: the Kobe-CONNECT Study. Hypertens Res 35, 1080–1086 (2012). https://doi.org/10.1038/hr.2012.110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2012.110