Abstract
We conducted a cross-sectional observation study that included 500 asymptomatic subjects to investigate the relationship between bone metabolism and coronary artery calcification (CAC) in hypertensive conditions. Osteoprotegerin (OPG) and osteopontin (OPN) levels and their associations with hypertension were analyzed to predict CAC in 316 subjects. Multislice computed tomography was used to quantify CAC. Multivariate analysis of variance was used to test the non-interactive effects of hypertension, CAC severity and biomarker levels, and the logistic regression model was applied to predict the risk of CAC. OPG and OPN concentrations were significantly higher in the hypertensive than the normotensive subjects, at 3.0 (2.3–4.0) pmol l−1 and 51 (21–136) ng ml−1 vs. 2.4 (2.0–3.0) pmol l−1 and 41 (13–63) ng ml−1, respectively. The OPG level, but not OPN level, increased with age (r=0.29; P=0.0001). Zero or minimal CAC (<10 Agatston units (AU)) was observed in 63% of the subjects, mild (11–100 AU) in 17%, moderate (101–400 AU) in 12% and severe (401–1000 AU)-to-extensive (>1000 AU) in 8%. In hypertensive subjects, only glomerular filtration rate (GFR) (β=−0.67) and gender (β=0.52) were significant predictors for CAC (R=0.68). In normotensive patients, GFR (β=−0.81), gender (β=0.48) and log-transformed OPG levels (β=0.15) were significant predictors for CAC. OPG levels were associated with an increased risk of CAC in normotensive subjects only (odds ratio: 3.37; 95% confidence interval (1.63–6.57); P=0.0002). OPG predicted a premature state of vascular calcification in asymptomatic normotensive individuals, and renal function significantly contributed to this process in both hypertensive and normotensive subjects.
Similar content being viewed by others
Introduction
Prime regulators of bone remodeling, such as osteoprotegerin (OPG), may substantially influence the severity of cardiovascular disease.1, 2, 3 Baseline levels of osteopontin (OPN) are an independent predictor of future adverse cardiac events in patients with chronic stable angina and may be useful for risk stratification.4 OPN and OPG are the glycoproteins implicated in the regulation of bone and vascular calcification. OPG acts as a soluble receptor secreted to inhibit the activity of the receptor activator of nuclear factor κ-B ligand.1, 5 OPN exerts an opposite influence on osteoclastogenesis by stimulating anti-apoptotic signaling.6 OPN and OPG are upregulated in tissues during atherosclerosis, valve stenosis and myocardial infarction,7, 8 which suggest that OPN and OPG may have a regulatory role in cardiac pathology and vascular remodeling.
The association between hypertension, imbalanced bone metabolism and coronary artery mineralization has recently been identified.9, 10, 11 Hypertension, antihypertensive treatment and OPG levels are univariate predictors of coronary artery calcification (CAC) progression.10, 12 In postmenopausal women, carotid atherosclerosis measured as arterial wall thickness is negatively correlated with lumbar spine bone mass, which suggests that bone metabolism may have a more significant impact on endothelial dysfunction and atherosclerosis progression than previously considered.11 Moreover, chronic vascular inflammation may lead to significant subclinical atherosclerosis and drive a positive feedback loop, resulting in vascular calcification.13
However, the role of hypertension in the progression of vascular mineralization has been poorly investigated. It is unclear whether human OPN and OPG levels are clinically associated with the progression or development of endothelial damage in asymptomatic individuals. Thus, we aimed to evaluate biomarkers that were potentially useful in predicting vascular damage and dysfunction in both asymptomatic individuals and those being treated for cardiovascular disease.
The aim of the present study was to clarify the relationship between the markers of bone metabolism and endothelial dysfunction, and to examine the association between plasma calcification markers and other clinical variables in patients with and without arterial hypertension. We hypothesized that OPN and OPG expression may predict endothelial dysfunction, influence vascular remodeling and lead to arteriosclerosis and/or calcification.
Methods
Subjects
The CRABIS Study (prevention program: CRacow Atherosclerosis Bioimaging Study) is a prospective, population-based survey to investigate the relationship between epidemiology and the pathogenesis of atherosclerosis and vascular calcification. In the 2002 baseline evaluation, asymptomatic subjects >18 years of age selected from southern Poland, all of Caucasian origin, were recruited using a population-based approach by cooperating with general practitioners. Information was obtained by self-reported questionnaires, physical examination, laboratory tests and a structured interview to investigate any family history of cardiovascular disease, including acute coronary syndrome, smoking and use of medications. Subjects were considered to have hypertension if they were taking antihypertensive agents or had a systolic blood pressure ⩾140 mm Hg and/or diastolic blood pressure ⩾90 mm Hg. The flow diagram shown in Figure 1 provides the methodology of patient enrollment. Ultimately, 338 patients were selected for the study.
Blood specimens
All blood specimens were taken 12 h after fasting. Lipid profiles were assayed by routine laboratory techniques, fibrinogen was determined using the Clauss method and high-sensitivity C-reactive protein (CRP) was measured with the use of the immunoturbidimetric method (Dimention, Xpand; Siemens, Erlangen, Germany). Serum creatinine was assessed using a non-kinetic alkaline picrate Jaffe's method (Dimention, Xpand; Siemens) with quality control afforded by the Randox International Quality Assessment Scheme (RIQAS). The glomerular filtration rate (GFR) was estimated using the abbreviated modification of diet in renal disease equation as recommended by the National Kidney Foundation as follows: estimated GFR (in ml min−1 per 1.73 m2)=186 × (serum creatinine (in mg dl−1)−1.154 × age−0.203 × 0.742 (if female) × 1.210 (if black)). Separated plasma and serum were stored at −80 °C until analysis. Plasma OPN (R&D, Wiesbaden-Nordenstadt, Germany) and serum OPG (Biomedica Medizinprodukte GmbH, Wien, Austria) levels were determined using commercially available immunoenzymatic assays. The limits of detection for the OPN and OPN assays were 0.14 pmol l−1 and 0.011 ng ml−1, respectively. The inter- and intra-assay coefficients of variation were <7.5%.
CAC imaging
The quantitative evaluation of calcification in coronary arteries was performed using multislice computed tomography. The Somatom Plus 4 Volume Zoom (Siemens) was utilized until the end of February 2003, and the Somatom Sensation 16 Cardiac (Siemens) was utilized from March 2003 to November 2004. The standardized 3 mm slice thickness protocol was performed according to the previously described methods.13, 14 Patients were considered to have coronary calcifications on multislice computed tomography images where more than two contiguous pixels of computed tomography attenuation >130 Hounsfield units (HU) were present. All values for the left main, left anterior descending, circumflex and right coronary arteries were added to calculate the total Agatston score for CAC. The quantitative analysis was performed by a single, experienced investigator blinded to the clinical data on the 3DLeonardo workstation using the Syngo CaScoring application (Siemens).14 The CAC scores were classified into five categories the basis of their severity: no calcified plaque (zero), 1–10 Agatston units (AU) (minimum), 11–100 AU (mild), 101–400 AU (moderate) and >400 AU (severe and extensive), as previously described.13
Statistical analysis
All analyses were carried out with Statistica version 8.0 (StatSoft Tulsa, OK, USA). The Kolmogorov-Smirnov one-sample test was performed to test the cumulative sample distributions. Continuous variables are presented as the mean±s.d. or median with interquartile range (Q1–Q3). Because the CAC distribution and some of the biomarkers were highly skewed, a log transformation was applied to normalize the distribution. To do this, a value of 1 was added to the total calcium score because many subjects had a total calcium score of 0. The multivariate regression model used to predict the CAC severity in hypertensive and normotensive subjects was adjusted for those parameters that displayed a significant difference by analysis of variance. The ordered logistic regression model was used to predict moderate and severe CAC in hypertensive and normotensive subjects, with values given as the odds ratio and the 95% confidence interval. Variables were compared using the unpaired t-test or the Mann–Whitney U-test, for continuous variables or by the Fisher's exact test or the χ2-test for categorical variables. Post hoc multivariate analysis of variance was used to test the hypothesis of non-interactive effects of hypertension treatment, CAC severity and concentrations of biomarkers. Bivariate correlations between biomarkers and age were performed with the Spearman rank correlation, and the log-transformed biomarkers were analyzed with the Pearson correlation test. P-values <0.05 were considered significant.
Results
Characteristics of the study groups
Ultimately, 316 asymptomatic subjects were included in the analysis: 134 (42.4%) men aged 57.0±7.9 years (min-max: 20 and 77 years) and 182 (57.6%) women aged 59.8±8.1 years (min-max: 40 and 82 years). Because some clinical data are influenced by gender, the differences between women and men were analyzed. Smoking status, age and biochemical parameters, including high-density cholesterol fraction, glucose, fibrinogen, CRP, creatinine and GFR, were different with respect to gender (Table 1). No differences were observed in OPG and OPN levels, diabetes, arterial hypertension or pharmacological treatment between the men and the women (Table 1). Antihypertensive drugs were used in 211 subjects (67%) in mono- (16%) or combination therapy (54%). These included angiotensin-converting enzyme inhibitors (59%), β-blockers (41%), diuretics (28%), calcium channel blockers (17%) and angiotensin II receptor antagonists (1.4%). There were no differences between the treatment of male and female patients. The hypertensive and normotensive subjects differed with respect to age, body mass index and a number of biochemical parameters, including OPG and OPN (Table 2).
OPG and OPN levels and cardiovascular risk factors
An analysis of the relationship between demographic parameters, as well as OPG and OPN concentrations, was performed. No significant differences were observed in OPG or OPN concentrations between the men and women (Table 1). The OPG and OPN concentrations were significantly higher in the hypertensive subjects in comparison with the normotensive subjects (Table 2). Diabetes did not influence the biomarkers analyzed in our study. In the diabetic subjects, the OPG and OPN concentrations were 3.1 (2.1–4.2) pmol l−1 and 75 (19–208) ng ml−1, respectively, whereas in the non-diabetic subjects, the concentrations were 2.9 (2.1–9.9) pmol l−1 and 46 (20–87) ng ml−1 (P=0.85; P=0.22), respectively. Despite the similarity between the diabetic and non-diabetic subjects with respect to OPG and OPN levels, a small association between the OPN and glucose levels in the total population was observed (r=0.13, P=0.017). This correlation was stronger in the normotensive subjects (r=0.33, P=0.01). Current smokers had similar concentrations of calcification biomarkers in comparison with the non-smokers; the OPG levels were 2.9 (2.2–3.5) pmol l−1 vs. 2.9 (2.2–4.0) pmol l−1 (P=0.74) and OPN levels were 48 (21–118) ng ml−1 vs. 46 (19–106) ng ml−1 (P=0.67) in smokers and non-smokers, respectively. OPG, but not OPN, levels correlated significantly with age: r=0.29 (P=0.0001) and r=0.02 (P=0.7), respectively. Similarly, CRP correlated with OPG (r=0.13, P=0.02), but not with OPN concentrations (r=0.05, P=0.4). Significant correlations of OPG with creatinine (r=0.13, P=0.02) and GFR (r=−0.21, P=0.0001) were observed. However, in hypertensive subjects only the correlation between GFR and OPG remained significant (r=−0.14, P=0.02). There were no correlations between the OPG and OPN levels with body mass index, at r=0.038 (P=0.5) and r=0.06 (0.28), respectively.
Distribution of calcification biomarkers in the study population
We also analyzed subjects with respect to CAC status. A total of 200 subjects (63%) were classified with a zero or minimal CAC score (<10 AU), including 154 and 46 patients, respectively. The remaining 116 patients (37%) had varying degrees of coronary calcification. CAC was mild in 52 patients (17%), moderate in 38 patients (12%) and severe/extensive in 26 patients (8%). The female population did not differ from the male population with respect to their CAC distribution; 126 (67%) women had zero or minimal CAC in comparison with 74 men (54%) with CAC <10 (χ2=1.44; P=0.23). However, the women had significantly lower calcification volumes than the men, at 0 (0–26) vs. 3.9 (0–160) AU (P=0.003), respectively. Hypertensive subjects had higher calcification values than normotensive subjects (Table 2). Additionally, analysis of variance of some risk factors and biochemical parameters with respect to the severity of CAC was performed (Table 3). Significant differences in age, glucose, creatinine and GFR parameters were observed between groups of subjects at different stages of coronary calcification.
Association between hypertension and calcification biomarkers
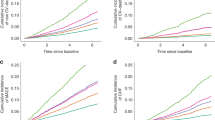
The distribution of OPG and OPN levels in the hypertensive and normotensive subjects is shown in Figure 2. A multivariate analysis of variance analysis of log-transformed OPG values with respect to CAC severity and hypertensive status showed that hypertension had a strong impact on OPG levels, both in subjects with zero and minimal arterial calcification (P=0.009) (Figure 2a). The normotensive subjects with zero or minimal CAC had significantly lower OPG levels in contrast to the subjects with moderate and severe calcification, at 2.2 (1.5–2.5) pmol l−1 and 3.3 (2.8–5.0) pmol l−1 (P<0.0001), respectively. In the hypertensive subjects, such a relationship was not observed, as OPG levels were 3.0 (2.4–4.0) and 3.1 (2.3–4.1) pmol l−1 (P=0.67). Moreover, normotensive subjects with minimal CAC (1) had lower OPG concentrations than hypertensive subjects, with values of 1.9 (1.1–2.8) pmol l−1 and 3.3 (2.9–3.7) pmol l−1 (P=0.003), respectively. These two groups were not significantly different with respect to age (P=0.8); however, normotensive subjects had a tendency for lower glucose concentrations compared with hypertensive patients, with values at 5.3 (4.9–5.8) mmol l−1 and 5.8 (5.5–6.0) mmol l−1 (P=0.07), respectively. An increase in the OPG level of normotensive subjects with severe calcification (3) was not significant when compared with hypertensive patients (4.4 (2.7–6.0) pmol l−1 vs. 3.6 (2.9–4.3) pmol l−1 (P=0.33), respectively). Normotensive and hypertensive subjects with severe calcification did not differ with respect to age, diabetes prevalence or glucose concentrations (P=0.8, P=0.2 and P=0.3, respectively). Those subjects undergoing treatment for hypertension had higher average OPG concentrations than untreated subjects (3.0 (2.3–4.0) pmol l−1 vs. 2.5 (2.1–3.5) pmol l−1 (P=0.007), respectively) (Figure 2c). A multivariate analysis of variance analysis of log-transformed OPG values with respect to CAC severity and hypertension treatment showed that moderate and severe arterial calcification had a higher impact on OPG than zero or minimal calcification (P=0.01) (Figure 2c).
Multivariate analysis of variance (MANOVA) of log-transformed OPG (a, c) and OPN (b, d) values in subjects with different stages of coronary calcification (CAC), respectively with and without hypertension (a, b) or hypertension treatment (c, d). 0—zero CAC; 1—minimum CAC (<10 AU); 2—mild CAC(11-100 AU); 3—moderate CAC (101–400 AU); 4—severe (401–1000 AU) and extensive (>1000 AU) CAC.
The subjects with moderate calcification demonstrated average OPN levels similar to those who had severe and extensive calcification, both in the normotensive and hypertensive group, at 39 (13–66) ng ml−1 vs. 43.4 (19–51) ng ml−1 (P=0.9) and 46 (20–127) ng ml−1 vs. 56 (21–163) ng ml−1 (P=0.41), respectively. However, a significant decrease in OPN was observed in normotensive subjects with minimal CAC compared with a zero or mild CAC, at values of 11 (7–16) ng ml−1 vs. 48 (17–66) ng ml−1 or 46 (10–47) ng ml−1 (P=0.002; P=0.002), respectively (Figure 2b). We found no difference in the OPN levels between the treated and untreated hypertensive subjects (46 (21–119) ng ml−1 vs. 47(17–81) ng ml−1, P=0.67, respectively).
In hypertensive subjects, the multivariate regressions model used to predict CAC severity included parameters such as age, gender, GFR, fibrinogen and OPG levels. The models showed that only GFR (β=−0.67) and gender (β=0.52) were significant predictors for CAC (R=0.68, P<0.00001). Interestingly, in normotensive subjects, GFR (β=−0.81), gender (β=0.48) and log-transformed OPG levels (β=0.15) were significant predictors for CAC.
In the logistic regression model, log-transformed fibrinogen levels were associated with an increased incidence of moderate and severe/extensive CAC in both hypertensive and normotensive subjects. OPG was associated with an increased risk of CAC in normotensive subjects only (Table 4).
Discussion
In our study, we observed that serum OPG is a predictor of moderate and severe CAC in normotensive and apparently healthy subjects. We also found a relationship between hypertension and bone metabolic biomarkers, as evidenced by increased OPG and OPN levels. Previous studies have shown that OPG and OPN levels are strong independent predictors of cardiovascular disease.15, 16 Our findings also indicated their essential role for the calcification processes in endothelial dysfunction, which resulted in the elevation of bone metabolic biomarkers in hypertensive subjects.9
The relationship between hypertension and calcification may involve several pathophysiological mechanisms. The most important is calcification of the arterial tunica media, which contributes to vascular stiffness and increases pulse-wave velocity while decreasing diastolic and increasing systolic blood pressure.17 Some data have suggested that arterial stiffness and atherosclerosis are related processes, and that pulse-wave velocity is strongly and independently associated with CAC and OPG.1, 18 In our study, we observed that hypertensive subjects had a higher calcification score than their normotensive counterparts. These data are in agreement with a previous study by van Popele et al.18 in which the highest increase in pulse-wave velocity in relation to calcification was observed in hypertensive subjects.
It has been shown that OPN overexpression has an essential role in modulating compensatory cardiac fibrosis and hypertrophy in carotid plaque formation in symptomatic patients.19, 20 Despite that angiotensin II blockade does not downregulate OPN secretion in vitro,20 Opn- knockout mice (OPN−/−) treated with angiotensin II did not develop hypertension or cardiac fibrosis, in contrast to wild-type mice.21 Moreover, OPN expression is involved in the coordinated regulation of the NFκB, ERK1/2 and AP-1 signaling pathways, which have been found to be involved in various pathologies, including immunological disorders, neoplastic transformation and renal disorders.22, 23 This pleiotropic pattern may explain why OPN is not a good predictor of vascular calcification specifically, despite that its presence was documented in calcified coronary artery plaques.8, 20 OPN is expressed in adipose tissue and in the livers of obese subjects; thus, obesity-induced inflammation may obscure OPN involvement in vascular calcification.24 We observed similar levels of OPN in diabetic and non-diabetic subjects, but we identified some associations between high glucose levels and OPN concentrations in our study population as a whole. A possible mechanism to explain this finding is that OPN expression is glucose dependent and upregulated in hyperglycemic conditions.25 This may also explain the relationship between increased glucose concentration and CAC severity.
Further, the greater relevance of our study is in showing that OPG levels were an independent predictor of CAC, but this association was significant in normotensive subjects only. OPG may contribute to the differentiation of inflammatory cells to osteoclast-like or osteoblast-like cells. The probable mechanism involves dendritic cell survival and expression of proinflammatory cytokines.26 However, this process is likely masked by different stimuli in hypertensive patients, which enhance OPG expression even in patients with zero or minimal CAC.27 It was previously observed that higher OPG concentrations were associated with an increased risk of all-cause mortality (odds ratio 1.39 (1.04–1.85)) and cardiovascular mortality (odds ratio 1.83 (1.10–3.05)).28 Hence, OPG testing could provide more information about a patient's status and could be used for risk stratification of future coronary events in the general population.29
In agreement with previous studies, we found that OPG correlated with age.15, 29 However, in contrast to some other studies, we did not observe increased values in the female subpopulation.15 In our study, women differed significantly from men regarding biochemical parameters, such as creatinine, GFR and fibrinogen levels, as well as CAC scores. It was previously documented that the median calcium scores in men are almost equal to the 75th percentile of calcium scores in women of the same age.30 One possible explanation is the role of dietary habits, including caloric intake, coffee or alcohol consumption. These data were presented in our previous study, which was a self-rated satisfaction of patients’ quality of life.31 However, we did not then observe a difference between OPG and OPN levels regarding gender. The limited number of subjects studied therein did not enable us to evaluate the variance between pre- and postmenopausal women; thus, we did not assume the role of sex hormones in the CAC score. Additionally, smoking status did not influence the OPG or OPN levels. It is possible that the influence of passive smoking or not providing accurate information about smoking behavior may have a role in this lack of association.
Inflammation is pivotally involved in atherosclerotic processes, which ultimately lead to plaque formation and CAC. OPG, a vascular calcification inhibitor, is upregulated when an inflammation process develops.26 Despite our not observing an increase in CRP levels with respect to the CAC score categories, a significant correlation between CRP and OPG was visible. Vascular calcification is a multistep process that is initiated by the differentiation of vascular cells of mesenchymal origin to osteoblast-like cells in atherosclerotic regions. OPG limits the interaction between receptor activator of nuclear factor κ-B ligand–receptor and activator of nuclear factor κ-B that takes place between dendritic cells, which can promote their survival and the elevated expression of proinflammatory cytokines.26 This process is regulated by the same pathways that mediate vascular inflammation and endothelial dysfunction.5, 22 It is possible that the role of fibrinogen in vascular calcification is strictly associated with inflammation.
In the process of vascular calcification, renal dysfunction and hyperparathyroidism cannot be ignored. Our study is a population-based study in which 80% of the participants typically had a zero or minimum (<10 AU) CAC score; a few patients had a mild CAC score. Similarly, renal function, assessed as a reduction of GFR, was mostly within reference values. However, in subjects with the most severe calcification scores, we observed GFR to be significantly reduced. In the Rotterdam Study of elderly individuals, the mean CAC score in the lowest GFR tertile was significantly higher than the mean CAC score in the highest tertile.32 Unfortunately, serum calcium and phosphate levels were not measured during this study.
Endothelial dysfunction is a systemic disorder influenced by a balance between atherogenic and atheroprotective factors. An imbalance can lead to hypertension and vascular calcification. In some multivariable analyses, low bone mineral density or OPG levels have been independently associated with the progression of endothelial dysfunction measured by strain-gauge plethysmography33 or increased brachial-ankle pulse wave velocity.1, 34 Surprisingly, we observed that hypertension treatment significantly reduced OPG levels in subjects with zero or minimal CAC; thus, OPG levels may decrease as a patient's inflammatory status improves. To our knowledge, this study is the first to examine both calcification biomarkers (OPG and OPN) with the synergistic use of cardiovascular imaging (calcium scoring). Our cross-sectional study combined non-invasive diagnostic methods to build cardiovascular physiology images (multislice computed tomography) and to expand the role of biomarkers in determining endothelial function. This approach increases the reliability and potential of these diagnostics.35, 36 Moreover, zero calcification does not exclude the risk of coronary artery stenosis in symptomatic patients37 or in asymptomatic subjects. Both the extent and composition of atherosclerotic plaques, as determined by multislice computed tomography, are prognostic for subsequent cardiac events.13 Thus, a synergistic diagnostic strategy (laboratory and imaging study) should be the focus to understand the mechanisms of disease and may be suitable in predicting future events.38 One further question is whether peripheral vascular function (calcification markers) correlates with CAC, and thus, whether the former may serve as a surrogate marker to identify individuals with coronary endothelial dysfunction.
Some limitations of our study merit consideration. First, our population consisted exclusively of subjects of Caucasian Polish descent. Therefore, the extrapolation of any conclusions from the present investigation may be inaccurate, and further studies of different clinical cohorts are needed to confirm and expand our findings. Second, we had limited data concerning renal function parameters. Depending on the GFR and creatinine levels in our group, it appears that our subjects did not have renal insufficiency; however, a significant relationship between GFR and hypertension was observed. Third, we limited our investigation to a clinical cohort of asymptomatic subjects with no evidence of CAD. Finally, 20% of the subjects of our study population had severe or extensive CAC. In our retrospective analysis, we neither investigated markers of cardiac and vascular injury, such as troponins or natriuretic peptides, nor measured brachial flow-mediated dilatation, which could be useful for the diagnosis of potential myocardial damage and vascular dysfunction.
We can conclude that OPG may be helpful in predicting premature states of vascular calcification in asymptomatic normotensive individuals, and that this information may be useful to provide a novel approach for the diagnosis of coronary artery disease. Additionally, we could assume that in asymptomatic subjects, vascular calcification is not related to endothelial function alone, but rather, that renal function also significantly contributes to this process.
References
Waluś-Miarka M, Katra B, Fedak D, Czarnecka D, Miarka P, Woźniakiewicz E, Małecki M, Idzior-Waluś B . Osteoprotegerin is associated with markers of atherosclerosis and body fat mass in type 2 diabetes patients. Int J Cardiol 2011; 147: 335–336.
Jono S, Ikari Y, Shioi A, Mori K, Miki T, Hara K, Nishizawa Y . Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation 2002; 106: 1192–1194.
Siepi D, Marchesi S, Vaudo G, Lupattelli G, Bagaglia F, Pirro M, Brozzetti M, Roscini AR, Mannarino E . Preclinical vascular damage in white postmenopausal women: the relevance of osteoprotegerin. Metabolism 2008; 57: 321–325.
Georgiadou P, Iliodromitis EK, Kolokathis F, Varounis C, Gizas V, Mavroidis M, Capetanaki Y, Boudoulas H, Kremastinos DT . Osteopontin as a novel prognostic marker in stable ischaemic heart disease: a 3-year follow-up study. Eur J Clin Invest 2010; 40: 288–293.
Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ . Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA 1999; 96: 3540–3545.
Abe M, Hiura K, Wilde J, Shioyasono A, Moriyama K, Hashimoto T, Kido S, Oshima T, Shibata H, Ozaki S, Inoue D, Matsumoto T . Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood 2004; 104: 2484–2491.
Fitzpatrick LA, Severson A, Edwards WD, Ingram RT . Diffuse calcification in human coronary arteries. Association of osteopontin with atherosclerosis. J Clin Invest 1994; 94: 1597–1604.
Tamura A, Shingai M, Aso N, Hazuku T, Nasu M . Osteopontin is released from the heart into the coronary circulation in patients with a previous anterior wall myocardial infarction. Circ J 2003; 67: 742–744.
Stępień E, Wypasek E, Stopyra K, Konieczyńska M, Przybyło M, Pasowicz M . Increased levels of bone remodeling biomarkers (osteoprotegerin and steopontin) in hypertensive individuals. Clin Biochem 2011; 44: 826–831.
Anand DV, Lim E, Darko D, Bassett P, Hopkins D, Lipkin D, Corder R, Lahiri A . Determinants of progression of coronary artery calcification in type 2 diabetes role of glycemic control and inflammatory/vascular calcification markers. J Am Coll Cardiol 2007; 50: 2218–2225.
Sumino H, Ichikawa S, Kasama S, Takahashi T, Sakamoto H, Kumakura H, Takayama Y, Kanda T, Murakami M, Kurabayashi M . Relationship between carotid atherosclerosis and lumbar spine bone mineral density in postmenopausal women. Hypertens Res 2008; 31: 1191–1197.
Mikami S, Hamano T, Fujii N, Nagasawa Y, Isaka Y, Moriyama T, Matsuhisa M, Ito T, Imai E, Hori M . Serum osteoprotegerin as a screening tool for coronary artery calcification score in diabetic pre-dialysis patients. Hypertens Res 2008; 31: 1163–1170.
Anand DV, Lahiri A, Lim E, Hopkins D, Corder R . The relationship between plasma osteoprotegerin levels and coronary artery calcification in uncomplicated type 2 diabetic subjects. J Am Coll Cardiol 2006; 47: 1850–1857.
Miszalski-Jamka T, Klimeczek P, Banyś R, Krupiński M, Nycz K, Bury K, Lada M, Pelberg R, Kereiakes D, Mazur W . The composition and extent of coronary artery plaque detected by multislice computed tomographic angiography provides incremental prognostic value in patients with suspected coronary artery disease. Int J Cardiovasc Imaging 2011 (in press; DOI:10.1007/s10554-011-9799-0).
Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W, Willeit J . Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation 2004; 109: 2175–2180.
Jono S, Otsuki S, Higashikuni Y, Shioi A, Mori K, Hara K, Hashimoto H, Ikari Y . Serum osteoprotegerin levels and long-term prognosis in subjects with stable coronary artery disease. J Thromb Haemost 2010; 8: 1170–1175.
Dao HH, Essalihi R, Bouvet C, Moreau P . Evolution and modulation of age-related medial elastocalcinosis: impact on large artery stiffness and isolated systolic hypertension. Cardiovasc Res 2005; 66: 307–317.
van Popele NM, Mattace-Raso FU, Vliegenthart R, Grobbee DE, Asmar R, van der Kuip DA, Hofman A, de Feijter PJ, Oudkerk M, Witteman JC . Aortic stiffness is associated with atherosclerosis of the coronary arteries in older adults: the Rotterdam Study. J Hypertens 2006; 24: 2371–2376.
Matsui Y, Jia N, Okamoto H, Kon S, Onozuka H, Akino M, Liu L, Morimoto J, Rittling SR, Denhardt D, Kitabatake A, Uede T . Role of Osteopontin in cardiac fibrosis and remodeling in angiotensin II-induced cardiac hypertrophy. Hypertension 2004; 43: 1195–1201.
Golledge J, McCann M, Mangan S, Lam A, Karan M . Osteoprotegerin and osteopontin are expressed at high concentrations within symptomatic carotid atherosclerosis. Stroke 2004; 35: 1636–1641.
Xie Z, Singh M, Singh K . Osteopontin modulates myocardial hypertrophy in response to chronic pressure overload in mice. Hypertension 2004; 44: 826–831.
Renault MA, Jalvy S, Potier M, Belloc I, Genot E, Dekker LV, Desgranges C, Gadeau AP . UTP induces osteopontin expression through a coordinate action of NFkappaB, activator protein-1, and upstream stimulatory factor in arterial smooth muscle cells. J Biol Chem 2005; 280: 2708–2713.
Lorenzen J, Krämer R, Kliem V, Bode-Boeger SM, Veldink H, Haller H, Fliser D, Kielstein JT . Circulating levels of osteopontin are closely related to glomerular filtration rate and cardiovascular risk markers in patients with chronic kidney disease. Eur J Clin Invest 2010; 40: 294–300.
Kiefer FW, Zeyda M, Gollinger K, Pfau B, Neuhofer A, Weichhart T, Säemann MD, Geyeregger R, Schlederer M, Kenner L, Stulnig TM . Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes 2010; 59: 935–946.
Nilsson-Berglund LM, Zetterqvist AV, Nilsson-Ohman J, Sigvardsson M, González Bosc LV, Smith ML, Salehi A, Agardh E, Fredrikson GN, Agardh CD, Nilsson J, Wamhoff BR, Hultgårdh-Nilsson A, Gomez MF . Nuclear factor of activated T cells regulates osteopontin expression in arterial smooth muscle in response to diabetes-induced hyperglycemia. Arterioscler Thromb Vasc Biol 2010; 30: 218–224.
Chino T, Draves KE, Clark EA . Regulation of dendritic cell survival and cytokine production by osteoprotegerin. J Leukoc Biol 2009; 86: 933–940.
Kim SM, Lee J, Ryu OH, Lee KW, Kim HY, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM . Serum osteoprotegerin levels are associated with inflammation and pulse wave velocity. Clin Endocrinol (Oxf) 2005; 63: 594–598.
Ueland T, Wilson SG, Amirul Islam FM, Mullin B, Devine A, Bollerslev J, Zhu K, Prince RL . A cohort study of the effects of serum OPG and OPG gene polymorphisms on cardiovascular mortality in elderly women. Clin Endocrinol (Oxf) 2009; 71: 828–833.
Semb AG, Ueland T, Aukrust P, Wareham NJ, Luben R, Gullestad L, Kastelein JJ, Khaw KT, Boekholdt SM . Osteoprotegerin and soluble receptor activator of nuclear factor-kappaB ligand and risk for coronary events: a nested case-control approach in the prospective EPIC-Norfolk population study 1993–2003. Arterioscler Thromb Vasc Biol 2009; 29: 975–980.
van Woudenbergh GJ, Vliegenthart R, van Rooij FJ, Hofman A, Oudkerk M, Witteman JC, Geleijnse JM . Coffee consumption and coronary calcification: the Rotterdam Coronary Calcification Study. Arterioscler Thromb Vasc Biol 2008; 28: 1018–1023.
Tlałka M, Pasowicz M, Kolasa-Trela R, Konieczyńska M, Klimeczek P, Misiak M, Wilkosz T . Self-rated health as a predictor of subclinical atherosclerosis measured by CCS among asymptomatic individuals (Abstract). World Congress of Cardiology, Barcelona, Spain, Sep 02-06. Eur Heart J 2006; 27: S387.
El Barzouhi A, Elias-Smale S, Dehghan A, Vliegenthart-Proença R, Oudkerk M, Hofman A, Witteman JC . Renal function is related to severity of coronary artery calcification in elderly persons: the Rotterdam study. PLoS One 2011; 6: e16738.
Sanada M, Taguchi A, Higashi Y, Tsuda M, Kodama I, Yoshizumi M, Ohama K . Forearm endothelial function and bone mineral loss in postmenopausal women. Atherosclerosis 2004; 176: 387–392.
Sumino H, Ichikawa S, Kasama S, Takahashi T, Kumakura H, Takayama Y, Kanda T, Sakamaki T, Kurabayashi M . Elevated arterial stiffness in postmenopausal women with osteoporosis. Maturitas 2006; 55: 212–218.
Jaffe AS . Key issues in the developing synergism between cardiovascular imaging and biomarkers. Clin Chem 2008; 54: 1432–1442.
Pasowicz M, Starzyk K, Klimeczek P, Stepien E, Tracz W . Comparative study of the elevated hs-C-reactive protein and lipid profile, the selected markers of atherosclerotic inflammation and coronary artery calcifications in the middle-aged men with no artery disease or diabetes. (Abstract) Congress of the European Society of Cardiology, Vienna, Austria, Aug 30-Sep 03. Eur Heart J 2003; 24: S574.
Gottlieb I, Miller JM, Arbab-Zadeh A, Dewey M, Clouse ME, Sara L, Niinuma H, Bush DE, Paul N, Vavere AL, Texter J, Brinker J, Lima JA, Rochitte CE . The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol 2010; 55: 627–634.
Yang Q, Lu S, Chen Y, Song X, Jin Z, Yuan F, Li H, Zhou Y, Chen F, Huo Y . Plasma osteoprotegerin levels and long-term prognosis in patients with intermediate coronary artery lesions. Clin Cardiol 2011; 34: 447–453.
Acknowledgements
The authors are grateful to the participants of the CRABIS Study. They thank all field workers, computer assistants and laboratory technicians from the John Paul II Hospital, especially E Wypasek for her skillful contribution to data collection, M Stygar and K Olbrych for their help with the logistics of acquiring the blood samples and M Tlałka for her assistance in the allocation of patients. The study was supported by the Malopolskie Province Self-government in Krakow and a Grant from Mieczysław Pasowicz. Mieczysław Pasowicz received a Grant from the Malopolskie Province Self-government in Krakow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Stępień, E., Fedak, D., Klimeczek, P. et al. Osteoprotegerin, but not osteopontin, as a potential predictor of vascular calcification in normotensive subjects. Hypertens Res 35, 531–538 (2012). https://doi.org/10.1038/hr.2011.231
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2011.231