Abstract
Both osteoporosis and high blood pressure are major diseases in aging populations. Recent studies demonstrated that some antihypertensive drugs reduced the risk of bone fracture in elderly patients. Although calcium channel blockers (CCB) are widely used as first-line antihypertensive agents, there is no evidence that they prevent osteoporosis. In this study, we investigated the effects of two types of CCB on bone metabolism: cilnidipine (L-/N-type CCB), which suppresses norepinephrine release from the sympathetic nerve, and amlodipine (L-type CCB). In ovariectomized female spontaneous hypertensive rats, administration of cilnidipine, but not amlodipine, resulted in a significant increase in the ratio of alkaline phosphatase to tartrate-resistant acid phosphatase (TRAP) and a decrease in the number of osteoclasts, as assessed by TRAP staining in the proximal tibia. Bone mineral density, moreover, was significantly higher in the cilnidipine group as compared with the amlodipine group and was associated with a significant decrease in a urinary collagen degradation product (deoxypyridinoline). The degree of prevention of osteoporosis by cilnidipine was similar to that of carvedilol (a β-blocker) because β-blockers reduce fracture risks though the inhibition of osteoclast activation. Interestingly, these effects cannot be attributed to the reduction of blood pressure because all three drugs significantly decreased blood pressure. In contrast, both cilnidipine and carvedilol, but not amlodipine, significantly decreased heart rate, indicating that both cilnidipine and carvedilol suppressed sympathetic nervous activity. Overall, our present data showed that cilnidipine (L-/N-type CCB) ameliorated osteoporosis in ovariectomized hypertensive rats. These pleiotropic effects of antihypertensive drugs such as cilnidipine and carvedilol might provide additional benefits in the treatment of hypertensive postmenopausal women.
Similar content being viewed by others
Introduction
Hypertension and osteoporosis may share the same genetic and environmental background. Several clinical studies demonstrated that high blood pressure might be a risk factor for bone fractures, probably owing to the secondary activation of the parathyroid gland through an increase in urinary calcium excretion.1, 2, 3, 4, 5 Several antihypertensive drugs, including thiazides, β-blockers and angiotensin-converting enzyme inhibitors, decreased the risk of bone fractures and increased bone mineral density (BMD) clinically.6, 7, 8, 9, 10 However, meta-analysis of observational studies on the effects of antihypertensive drugs on fracture outcomes demonstrated no significant association between fractures and calcium channel blockers (CCBs).11, 12, 13
CCBs are divided into several subtypes, and non-dihydropyridine-type CCBs, but not dihydropyridine-type CCBs, are reported to reduce the risk of bone fractures through the inhibition of hyperparathyroidism-induced calcium uptake into osteoblasts and an elevation of intracellular calcium in osteoclasts.14, 15 Cilnidipine, a new type of CCB that blocks the N-type calcium channel and the L-type calcium channel, is therefore thought to affect bone metabolism, possibly through the inhibition of both the sympathetic nervous system and the voltage-dependent calcium channel.16, 17, 18, 19 β-blockers are known to affect the relationship between the sympathetic nervous system and bone metabolism, leading to increased BMD.20, 21 Thus, in this study, we investigated the effects of cilnidipine on bone metabolism in comparison with those of amlodipine, an L-type dihydropyridine CCB, and carvedilol, a β-blocker that is known to reduce fracture risk though the inhibition of osteoclast activation via β2-adrenergic receptors, in an ovariectomy-induced rat osteoporosis model.
Methods
Anti-hypertensive drugs
Cilnidipine was kindly provided by the Mochida Pharmaceutical (Tokyo, Japan), carvedilol was kindly provided by the Daiichi Sankyo (Tokyo, Japan) and amlodipine was purchased from Sigma (St Louis, MO, USA).
Rat ovariectomy osteoporosis model
All experiments were approved by the Ethical Committee for animal experiments at Osaka University Graduate School of Medicine. Female adult spontaneous hypertensive rats (SHR) (10 weeks old) were purchased from SLC Japan (Shizuoka, Japan). After the rats were anesthetized with intraperitoneal ketamine (80 mg kg−1) and xylazine (10 mg kg−1), a bilateral ovariectomy (OVX) or a sham operation was performed. Cilnidipine, amlodipine or carvedilol was administered with drinking water (3 mg kg−1 per day). The body weight of these rats was recorded for 4 weeks. At 4 weeks after the operation, systolic blood pressure was measured using the tail-cuff method (BP-98A, Softron, Koenji, Tokyo, Japan), and urinary deoxypyridinoline was measured by EIA (Metra Biosystems, Mountain View, CA, USA). The rats were then anesthetized and killed to collect the femurs, tibias and blood for biochemical analysis.
Alkaline phosphatase and tartrate-resistant acid phosphatase activity
Alkaline phosphatase (ALP) activity was measured to evaluate osteoblast activity, whereas tartrate-resistant acid phosphatase (TRAP) activity was measured to evaluate osteoclast activity, as previously described.22 The proximal tibias and distal femurs were excised and homogenized in a 10 mmol l−1 triethanolamine buffer (pH 7.5) for TRAP and in a diethanolamine buffer (pH 9.8) for ALP. Supernatants were used to measure TRAP activity.23 For ALP activity, the supernatants were incubated using p-nitrophenylphosphate as a substrate for 30 min at 25°C, and absorbance was measured at 405 nm and converted into units (U: 1 U indicates the release of 1 mmol l−1 p-nitrophenol per min). The urinary deoxypyridinoline level was measured by EIA on day 28 of the experiment. Serum calcium, phosphate, ALP, TRAP and urinary calcium were measured on day 28.
Urinary calcium and deoxypyridinoline
Calcium was measured by the o-cresolphthalein complexone method using a commercial kit (Wako Diagnostic, Osaka, Japan). The concentration of the deoxypyridinoline in urine was determined by EIA using a Pyrilinks D assay kit (Metra Biosystems). The data were adjusted by creatinine.
Quantification of TRAP-positive stained area in proximal tibia
Proximal tibias separated from surrounding soft tissue were fixed in a 4% phosphate-buffered paraformaldehyde followed by decalcification with EDTA. Following graded ethanol dehydration, samples were embedded in paraffin, and 4–6 μm paraffin sections were prepared. To identify mature osteoclasts in the proximal tibia, TRAP staining was performed using a commercial staining kit (Primary Cell, Ishikari, Japan). Quantification of the TRAP-stained area per 0.3 × 0.3 mm2 under the growth plate of the proximal tibia was performed using NIH ImageJ.
Dual-energy X-ray absorptiometry
Bone density was measured by dual-energy X-ray absorptiometry bone densitometry (GE-Lunar DPX-IQ, GE Healthcare, Madison, WI, USA). High- and low-beam energy for all scans was 80 and 35 kV, respectively, at 0.5 mA, as previously described.18 BMD was obtained in g cm−2.
Statistical analysis
Statistical analysis was performed using Stat-View 5.0. software (SAS Institute, Cary, NC, USA). All results were expressed as mean±s.e.m. Data were compared using the analysis of variance followed by Dunnett's test for pair-wise comparisons. Values of P<0.05 were considered to be statistically significant.
Results
Cilnidipine and carvedilol ameliorated OVX-induced osteoporosis
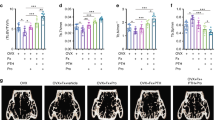
To elucidate the effects of antihypertensive drugs on bone metabolism, we employed an OVX model of estrogen deficiency in SHR.22 At 28 days after bilateral OVX, both ALP and TRAP activity were increased in the tibia of ovariectomized SHR as compared with those of the sham-operated SHR (Figures 1a and b). As TRAP activity was significantly increased more than ALP activity, the ratio of ALP to TRAP was markedly decreased in the tibia of ovariectomized SHR as compared with sham-operated rats (Figure 1c). In this model, the OVX-induced decrease in the ratio of ALP to TRAP activity was significantly ameliorated by cilnidipine, but not amlodipine (Figure 1c). Indeed, the TRAP-positive stained area was increased in the tibia of ovariectomized SHR, whereas treatment with cilnidipine, but not amlodipine, significantly decreased the TRAP-positive stained area (Figures 2a and b).
Effects of amlodipine, cilnidipine or carvedilol on alkaline phosphatase (ALP) and tartrate-resistant acid phosphatase (TRAP) activity in ovariectomized spontaneous hypertensive. (a) ALP activity (U per bone), (b) TRAP activity (U per bone), (c) ratio of ALP to TRAP activity. Sham, sham operation; OVX, bilateral ovariectomy; amlodipine: OVX+amlodipine (3 mg kg−1 per day), cilnidipine: OVX+cilnidipine (3 mg kg−1 per day), carvedilol: OVX+carvedilol (3 mg kg−1 per day). †P<0.05 vs. sham, *P<0.05 vs. OVX, N=6–10 per group.
Tartrate-resistant acid phosphatase (TRAP) staining of proximal tibia at 28 days in spontaneous hypertensive rats. (a) Representative pictures of TRAP staining in proximal tibia. Bar indicates 0.3 mm. (b) Quantification of TRAP-positive stained area under growth plate of proximal tibia per field (0.3 × 0.3 mm2). Sham=sham operation; OVX=bilateral ovariectomy; OVX+amlodipine: amlodipine treatment (3 mg kg−1 per day) with bilateral ovariectomy, OVX+cilnidipine: cilnidipine treatment (3 mg kg−1 per day) with bilateral ovariectomy, OVX+carvedilol: carvedilol treatment (3 mg kg−1 per day) with bilateral ovariectomy. †P<0.05 vs. sham, *P<0.05 vs. OVX, N=6–10 per group.
Importantly, these beneficial effects of cilnidipine were accompanied by a significant increase in BMD in ovariectomized SHR (as assessed by dual-energy X-ray absorptiometry), whereas amlodipine did not increase BMD (Figure 3a). Consistently, the increase in urinary deoxypyridinoline, a collagen degradation product that reflects bone loss, was significantly attenuated by cilnidipine, but not by amlodipine (Figure 3b). Similarly, although urinary calcium and serum phosphate were increased in ovariectomized SHR, treatment with cilnidipine, but not amlodipine, attenuated the ovariectomy-induced increase in urinary calcium and serum phosphate, as shown in Table 1. In contrast, serum calcium was not changed in either group (Table 1). Prevention of osteoporosis by cilidipine was similar to that of carvedilol (Figures 1, 2, 3).
Effects of amlodipine, cilnidipine and carvedilol on bone mineral density (BMD) and urinary deoxypyridinoline secretion in ovariectomized spontaneous hypertensive rats. (a) BMD by dual energy X-ray absorptiometry, (b) urinary deoxypyridinoline after 28 days. Sham=sham operation; OVX=bilateral ovariectomy; +amlodipine: amlodipine treatment (3 mg kg−1 per day) with bilateral ovariectomy, +cilnidipine: cilnidipine treatment (3 mg kg−1 per day) with bilateral ovariectomy, +carvedilol: carvedilol treatment (3 mg kg−1 per day) with bilateral ovariectomy. †P<0.05 vs. sham, *P<0.05 vs. OVX, N=6–10 per group.
It is noteworthy that the prevention of osteoporosis was independent of the blood pressure-lowering effects of cilnidipine whereas the administration of cilnidipine, carvedilol and amlodipine each significantly decreased blood pressure to the same degree compared with the sham group, only cilnidipine and carveilol had beneficial effects on bone metabolism, as shown in Table 2. There was no difference in body weight among all groups. Interestingly, cilnidipine and carvedilol treatment significantly reduced heart rates whereas amlodipine did not. These results indicate a suppression of adrenergic stimulation of the sympathetic nervous system by cilnidipine and carvedilol (Table 2).
Discussion
Given the increasing number of the elderly, treatments that can prevent aging-related diseases are crucial. Our group has focused on the relationship between hypertension and osteoporosis, which are two common diseases in the elderly population caused by the interaction of genetic and environmental factors. Currently, 50% of the hypertensive population comprises postmenopausal women, who are at high risk of osteoporosis.1, 24 Animal and epidemiological evidence suggests that high blood pressure is associated with abnormalities in calcium metabolism, leading to an increase in calcium loss, secondary activation of the parathyroid gland and increased movement of calcium from the bone, thereby increasing the risk of osteoporosis.25, 26 There is clinical evidence that several classes of antihypertensive drugs decrease the risk of bone fractures and increase BMD, whereas other antihypertensive drugs do not. Thus, it is important to ascertain which antihypertensive drug might prevent osteoporosis. In this study, we demonstrated that cilnidipine (L-/N-type CCB) and carvedilol ameliorate osteoporosis in ovariectomized hypertensive rats through the inhibition of osteoclast activation.
Interestingly, there is little clinical evidence of the prevention of osteoporosis by CCBs, although angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are known to prevent osteoporosis. CCBs, however, can be divided into several subtypes. Cilnidipine, which is reported to protect against cardiovascular27, 28, 29 and kidney disease,30, 31, 32 works in a variety of ways, including blocking the N-type calcium channel and the L-type calcium channel. Recently, cilnidipine has also been reported to suppress renin activity and inhibit the renal renin–angiotensin system. Distinct from amlodipine, a typical dihydropyridine type of CCB, cilnidipine reduced noradrenaline content in the renal cortex in salt hypertensive rats.32 Importantly, in a clinical study comparing cilnidipine and amlodipine in combination with an angiotensin receptor blocker, cilnidipine demonstrated superiority in preventing the progression of proteinuria in hypertensive patients.33 These results might be due to the inhibition of the renin–angiotensin system activation induced by the angiotensin receptor blocker. Such inhibition is partly explained by cilnidipine's sympatholytic action mediated by the N-type calcium channel blockade with a reduction in renin secretion by juxtaglomerular cells through renal sympathetic innervation. Because the inhibition of renin–angiotensin system with angiotensin receptor blockers and angiotensin-converting enzyme inhibitors in experimental models prevented osteoporosis in ovariectomy-induced bone loss,22, 34, 35 both the inhibition of renin–angiotensin system and the adrenergic stimulation by cilnidipine might have cumulative benefits on bone metabolism.
The relationship between sympathetic nerve activation and bone metabolism has been recently highlighted.20, 36, 37 Bone and its periosteum receive a rich supply of sensory and sympathetic nerves,38 suggesting the neuroendocrine regulation of bone remodeling. Both osteoblasts and osteoclasts possess α1 and α2 and/or β2-adrenergic receptors, and adrenergic stimulation from sympathetic nerves enhanced bone resorption.39, 40 One of the neuroendocrine hormones acting between the sympathetic nervous system and osteoporosis is leptin. Leptin may act on a population of neurons located in the ventromedial hypothalamus, which in turn stimulate the activity of intraosseous sympathetic nerve fibers. These fibers release norepinephrine, which binds to adrenergic receptors expressed on osteoblasts, thereby inhibiting their bone-forming activity. Indeed, a mouse model of leptin deficiency (ob/ob) and mutation of the leptin receptor (db/db) show increased bone mass, more specifically, increased trabecular bone volume. Because the sympathetic nervous system is also activated in hypertension, insulin resistance and metabolic syndrome (all of which may be associated with bone metabolism), the use of β-blockers to treat hypertensive patients with osteoporosis has been highlighted. Indeed, our study demonstrated that carvedilol also ameliorates osteoporosis in ovariectomized hypertensive rats.
Overall, our present data showed that cilnidipine, a new type of CCB that blocks the N-type calcium channel and the L-type calcium channel, ameliorated osteoporosis in ovariectomized hypertensive rats. These pleiotropic effects of antihypertensive drugs such as cilnidipine and carvedilol might benefit hypertensive postmenopausal women, as osteoporosis is the main cause of bone fractures in postmenopausal women as well as the elderly. Further studies to find new therapeutic uses for antihypertensive drugs beyond their blood pressure-lowering effects are necessary for the treatment of elderly hypertensive patients, especially women.
References
Cappuccio FP, Meilahn E, Zmuda JM, Cauley JA . High blood pressure and bone-mineral loss in elderly white women: A prospective study. Study of osteoporotic fractures research group. Lancet 1999; 354: 971–975.
Tsuda K, Nishio I, Masuyama Y . Bone mineral density in women with essential hypertension. Am J Hypertens 2001; 14: 704–707.
Imaoka M, Morimoto S, Kitano S, Fukuo F, Ogihara T . Calcium metabolism in elderly hypertensive patients: Possible participation of exaggerated sodium, calcium and phosphate excretion. Clin Exp Pharmacol Physiol 1991; 18: 631–641.
McCarron DA, Pingree PA, Rubin RJ, Gaucher SM, Molitch M, Krutzik S . Enhanced parathyroid function in essential hypertension: a homeostatic response to a urinary calcium leak. Hypertension 1980; 2: 162–168.
Perez-Castrillon JL, Justo I, Silva J, Sanz A, Igea R, Escudero P, Pueyo C, Diaz C, Hernandez G, Duenas A . Bone mass and bone modelling markers in hypertensive postmenopausal women. J Hum Hypertens 2003; 17: 107–110.
Reid IR, Ames RW, Orr-Walker BJ, Clearwater JM, Horne AM, Evans MC, Murray MA, McNeil AR, Gamble GD . Hydrochlorothiazide reduces loss of cortical bone in normal postmenopausal women: A randomized controlled trial. Am J Med 2000; 109: 362–370.
Schoofs MW, van der Klift M, Hofman A, de Laet CE, Herings RM, Stijnen T, Pols HA, Stricker BH . Thiazide diuretics and the risk for hip fracture. Ann Intern Med 2003; 139: 476–482.
Schlienger RG, Kraenzlin ME, Jick SS, Meier CR . Use of beta-blockers and risk of fractures. JAMA 2004; 292: 1326–1332.
Wasnich R, Davis J, Ross P, Vogel J . Effect of thiazide on rates of bone mineral loss: A longitudinal study. BMJ 1990; 301: 1303–1305.
Rejnmark L, Vestergaard P, Mosekilde L . Reduced fracture risk in users of thiazide diuretics. Calcif Tissue Int 2005; 76: 167–175.
Rejnmark L, Vestergaard P, Mosekilde L . Treatment with beta-blockers, ace inhibitors, and calcium-channel blockers is associated with a reduced fracture risk: a nationwide case-control study. J Hypertens 2006; 24: 581–589.
Albers MM, Johnson W, Vivian V, Jackson RD . Chronic use of the calcium channel blocker nifedipine has no significant effect on bone metabolism in men. Bone 1991; 12: 39–42.
Zacharieva S, Shigarminova R, Nachev E, Kamenov Z, Atanassova I, Orbetzova M, Stoynev A, Doncheva N, Borissova AM . Effect of amlodipine and hormone replacement therapy on blood pressure and bone markers in menopause. Methods Find Exp Clin Pharmacol 2003; 25: 209–213.
Boesgaard S, Hyldstrup L, Feldstedt M . Changes in calcium homoeostasis and bone formation in patients recovering from acute myocardial infarction: Effect of verapamil treatment. Danish study group on verapamil in myocardial infarction. Eur J Clin Pharmacol 1991; 41: 521–523.
Guggino SE, Lajeunesse D, Wagner JA, Snyder SH . Bone remodeling signaled by a dihydropyridine- and phenylalkylamine-sensitive calcium channel. Proc Natl Acad Sci USA 1989; 86: 2957–2960.
Hirning LD, Fox AP, McCleskey EW, Olivera BM, Thayer SA, Miller RJ, Tsien RW . Dominant role of n-type ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science 1988; 239: 57–61.
Takahara A, Fujita S, Moki K, Ono Y, Koganei H, Iwayama S, Yamamoto H . Neuronal ca2+ channel blocking action of an antihypertensive drug, cilnidipine, in imr-32 human neuroblastoma cells. Hypertens Res 2003; 26: 743–747.
Hayashi K, Wakino S, Sugano N, Ozawa Y, Homma K, Saruta T . Ca2+ channel subtypes and pharmacology in the kidney. Circ Res 2007; 100: 342–353.
Takahara A . Cilnidipine: A new generation ca channel blocker with inhibitory action on sympathetic neurotransmitter release. Cardiovasc Ther 2009; 27: 124–139.
Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G . Leptin regulates bone formation via the sympathetic nervous system. Cell 2002; 111: 305–317.
Pasco JA, Henry MJ, Sanders KM, Kotowicz MA, Seeman E, Nicholson GC . Beta-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong osteoporosis study. J Bone Miner Res 2004; 19: 19–24.
Shimizu H, Nakagami H, Osako MK, Hanayama R, Kunugiza Y, Kizawa T, Tomita T, Yoshikawa H, Ogihara T, Morishita R . Angiotensin II accelerates osteoporosis by activating osteoclasts. FASEB J 2008; 22: 2465–2475.
Shimizu H, Nakagami H, Tsukamoto I, Morita S, Kunugiza Y, Tomita T, Yoshikawa H, Kaneda Y, Ogihara T, Morishita R . Nfkappab decoy oligodeoxynucleotides ameliorates osteoporosis through inhibition of activation and differentiation of osteoclasts. Gene Ther 2006; 13: 933–941.
Nuzzo A, Rossi R, Modena MG . Hypertension alone or related to the metabolic syndrome in postmenopausal women. Expert Rev Cardiovasc Ther 2010; 8: 1541–1548.
Resnick LM, Laragh JH, Sealey JE, Alderman MH . Divalent cations in essential hypertension. Relations between serum ionized calcium, magnesium, and plasma renin activity. N Engl J Med 1983; 309: 888–891.
Cappuccio FP, Kalaitzidis R, Duneclift S, Eastwood JB . Unravelling the links between calcium excretion, salt intake, hypertension, kidney stones and bone metabolism. J Nephrol 2000; 13: 169–177.
Varagic J, Susic D, Frohlich ED . Cilnidipine improves spontaneously hypertensive rat coronary hemodynamics without altering cardiovascular mass and collagen. J Hypertens 2002; 20: 317–322.
Sakata K, Yoshida H, Tamekiyo H, Obayashi K, Nawada R, Doi O, Mori N . Comparative effect of clinidipine and quinapril on left ventricular mass in mild essential hypertension. Drugs Exp Clin Res 2003; 29: 117–123.
Nagai H, Minatoguchi S, Chen XH, Wang N, Arai M, Uno Y, Lu C, Misao Y, Onogi H, Kobayashi H, Takemura G, Maruyama R, Fujiwara T, Fujiwara H . Cilnidipine, an n+l-type dihydropyridine ca channel blocker, suppresses the occurrence of ischemia/reperfusion arrhythmia in a rabbit model of myocardial infarction. Hypertens Res 2005; 28: 361–368.
Fujita T, Ando K, Nishimura H, Ideura T, Yasuda G, Isshiki M, Takahashi K . Antiproteinuric effect of the calcium channel blocker cilnidipine added to renin-angiotensin inhibition in hypertensive patients with chronic renal disease. Kidney Int 2007; 72: 1543–1549.
Zhou X, Ono H, Ono Y, Frohlich ED . N- and l-type calcium channel antagonist improves glomerular dynamics, reverses severe nephrosclerosis, and inhibits apoptosis and proliferation in an l-name/shr model. J Hypertens 2002; 20: 993–1000.
Toba H, Yoshida M, Tojo C, Nakano A, Oshima Y, Kojima Y, Noda K, Wang J, Kobara M, Nakata T . L/n-type calcium channel blocker cilnidipine ameliorates proteinuria and inhibits the renal renin-angiotensin-aldosterone system in deoxycorticosterone acetate-salt hypertensive rats. Hypertens Res 2011; 34: 521–529.
Aritomi S, Niinuma K, Ogawa T, Konda T, Nitta K . Effects of an n-type calcium antagonist on angiotensin ii-renin feedback. Am J Nephrol 2011; 33: 168–175.
Shimizu H, Nakagami H, Osako MK, Nakagami F, Kunugiza Y, Tomita T, Yoshikawa H, Rakugi H, Ogihara T, Morishita R . Prevention of osteoporosis by angiotensin-converting enzyme inhibitor in spontaneous hypertensive rats. Hypertens Res 2009; 32: 786–790.
Shimizu H, Nakagami H, Yasumasa N, Osako MK, Shimamura M, Miyake T, Nakagami F, Koriyama H, Rakugi H, Morishita R . Combination of thiazide diuretics and angiotensin receptor blockers attenuates osteoporosis in hypertensive rats. Immun Endocr Metab Agents Med Chem 2010; 10: 115–122.
Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G . Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell 2000; 100: 197–207.
Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G . Leptin regulation of bone resorption by the sympathetic nervous system and cart. Nature 2005; 434: 514–520.
Hohmann EL, Elde RP, Rysavy JA, Einzig S, Gebhard RL . Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science 1986; 232: 868–871.
Moore RE, Smith II CK, Bailey CS, Voelkel EF, Tashjian Jr AH . Characterization of beta-adrenergic receptors on rat and human osteoblast-like cells and demonstration that beta-receptor agonists can stimulate bone resorption in organ culture. Bone Miner 1993; 23: 301–315.
Bowler WB, Gallagher JA, Bilbe G . G-protein coupled receptors in bone. Front Biosci 1998; 3: d769–d780.
Acknowledgements
This work was partially supported by the National Institute of Biomedical Innovation, a Grant-in-Aid from the Ministry of Public Health and Welfare, a Grant-in-Aid from Japan Promotion of Science, and by Special Coordination Funds from the Ministry of Education, Culture, Sports, Science and Technology, the Japanese Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Shimizu, H., Nakagami, H., Yasumasa, N. et al. Cilnidipine, but not amlodipine, ameliorates osteoporosis in ovariectomized hypertensive rats through inhibition of the N-type calcium channel. Hypertens Res 35, 77–81 (2012). https://doi.org/10.1038/hr.2011.143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2011.143
Keywords
This article is cited by
-
The Role of Osteogenic Effect and Vascular Function in Bone Health in Hypertensive Rats: A Study of Anti-hypertensive and Hemorheologic Drugs
Calcified Tissue International (2023)
-
Differential impact of antihypertensive drugs on cardiovascular remodeling: a review of findings and perspectives for HFpEF prevention
Hypertension Research (2022)
-
Links Between Hypertension and Osteoporosis: Benidipine Ameliorates Osteoporosis in Ovariectomized Hypertensive Rats Through Promotion of Osteoblast Proliferation and Inhibition of Osteoclast Differentiation
Current Cardiovascular Risk Reports (2012)