Abstract
Angiotensin receptor blockers (ARBs) or T- and L-type calcium channel blockers (CCBs) are useful for glomerular protection; however, the protective effects of combination therapy remain unclear. In this study, Dahl salt-sensitive rats were fed a high-salt diet and were treated daily with placebo, irbesartan (60 mg kg−1), efonidipine (30 mg kg−1), irbesartan (60 mg kg−1)+efonidipine (30 mg kg−1), amlodipine (3 mg kg−1), or irbesartan (60 mg kg−1)+amlodipine (3 mg kg−1) for 4 weeks. Significant reductions in systolic blood pressure were seen in the irbesartan-, efonidipine- and amlodipine-treated groups compared with the placebo-treated group; a further significant reduction was seen in the irbesartan+efonidipine-treated group compared with the irbesartan-treated group. Compared with the placebo-treated group, proteinuria was significantly lower in the irbesartan- and efonidipine-treated groups, but not in the amlodipine-treated group. Furthermore, a significant attenuation of proteinuria in the irbesartan+efonidipine-treated group compared with the irbesartan-treated group was observed; this effect was not observed in the irbesartan+amlodipine-treated group. The glomerulosclerosis index was significantly attenuated by all active treatments except amlodipine. The glomerulosclerosis index in the irbesartan+efonidipine-treated group, but not in the irbesartan+amlodipine-treated group, was significantly lower than that in the irbesartan-treated group. Significant attenuations of gene expressions of p22phox, transforming growth factor-β, monocyte chemoattractant protein-1 and collegen I were observed in the irbesartan- and efonidipine-treated groups, but not in the amlodipine-treated group. Values for these parameters were reduced to control levels in the irbesartan+efonidipine-treated group. Combination therapy with ARB and T- and L-type CCB might produce a powerful renal protective effect.
Similar content being viewed by others
Introduction
Kidneys have an important role not only in the homeostasis of water and electrolyte balance but also in the regulation of blood pressure. Angiotensin II is a crucial peptide for regulating blood pressure by slow and rapid pressor responses. The main functions of slow response are augmentation of sodium reabsorption in the proximal tubules and release of aldosterone from the adrenal cortex, whereas the main functions of rapid response are direct contractile response in peripheral resistance arteries and enhancement of peripheral noradrenergic neurotransmission. Therefore, blockade of angiotensin II is a useful antihypertensive strategy. In addition, angiotensin II directly increases mesangial matrix formation by stimulating transforming growth factor (TGF)-β expression.1 Inhibition of angiotensin II function induces dilation of efferent arterioles, and this mechanism contributes to amelioration of glomerular hypertension, resulting in a decrease in mesangial matrix formation.2 Therefore, blockade of angiotensin II is also useful for preventing glomerular injury.
Calcium channel blockers (CCBs), such as angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers (ARBs), have been widely used as antihypertensive agents. CCBs have favorable effects not only on hypertension but also on cardiovascular disease. On the other hand, various CCBs have been shown to have different effects on glomerular injury.3 There are three types of CCBs: L-type, T-type and N-type. The different functions of these calcium channel subtypes may result in different effects on glomerular injury. In general, L-type CCBs, such as nifedipine and amlodipine, preferentially dilate afferent, but not efferent, arterioles.4 In contrast, T- and L-type CCBs, such as efonidipine and mibefradil, dilate both afferent and efferent arterioles in vitro and in vivo.5, 6 For example, although efonidipine was shown to markedly prevent an increase in proteinuria in subtotally nephrectomized spontaneously hypertensive rats, nifedipine did not reduce proteinuria despite leading to a similar reduction in systolic blood pressure (SBP).7
Dahl salt-sensitive (DS) rats are a paradigm of human salt-sensitive hypertension. In DS rats, components of the renin–angiotensin system, including angiotensinogen and angiotensin II levels and angiotensin II type 1 receptor gene expression in kidneys, are elevated.8 ARBs attenuate angiotensin II function through angiotensin II type 1 receptors, but they are known to increase renin secretion from juxtaglomerular cells by a feedback mechanism. This feedback mechanism may attenuate the blockade of angiotensin II by ARBs. T- and L-type CCBs attenuate renin secretion and renin gene expression in conscious rats, whereas L-type CCBs do not.9 In the present study, we evaluated the efficacy of the combination of an ARB (irbesartan) and either a T- and L-type CCB (efonidipine) or an L-type CCB (amlodipine) in DS rats.
Methods
Animals
Six-week-old male DS rats were obtained from Japan SLC (Shizuoka, Japan). A total of 48 DS rats were fed a high-salt diet (8% NaCl) and were orally administered placebo (n=8), irbesartan (60 mg kg−1 per day) (n=8), efonidipine (30 mg kg−1 per day) (n=8), irbesartan (60 mg kg−1 per day)+efonidipine (30 mg kg−1 per day) (n=8), amlodipine (3 mg kg−1 per day) (n=8), or irbesartan (60 mg kg−1 per day)+amlodipine (3 mg kg−1 per day) (n=8) for 4 weeks. As normal controls, DS rats were fed a normal salt diet (0.21% NaCl) (n=8). Urinary excretion for 24 h was collected in individual metabolic cages, and urinary protein excretion was determined. Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) excretion for 24 h was determined using an 8-OHdG enzyme-linked immunosorbent assay kit (Japan Institute for the Control of Aging, Shizuoka, Japan). SBP was monitored by tail-cuff plethysmography (BP-98, Softron Tokyo, Japan). After 4 weeks, the body weights of the rats were measured. The animals were anesthetized with 35 mg kg−1 of sodium pentobarbital intraperitoneally, and then blood and tissue samples were obtained. The experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Animal Research Laboratory, Osaka Medical College).
Renin activity, angiotensin II concentration and aldosterone concentration in blood
Plasma renin activity (PRA) was determined using a radioimmunoassay kit (DiaSorin, Stillwater, MN, USA). Serum angiotensin II concentration was measured using an enzyme immunoassay kit (Peninsula Laboratories, San Carlos, CA, USA). Plasma aldosterone concentration was measured using a SPAC-S aldosterone kit (TFB, Tokyo, Japan).
Histological examination
Kidney tissue specimens were fixed with 10% buffered formalin solution. The fixed kidney tissues were embedded in paraffin and then cut to a thickness of 3 μm.
To evaluate glomerulosclerosis scores, sections were stained with periodic acid Schiff. We evaluated a minimum of 100 glomeruli per specimen. The severity of lesions was graded from 0 to 4+ according to the percentage of glomerular involvement: 0, no lesions; 1+, less than 25%; 2+, 26 to 50%; 3+, 51 to 75%; 4+, more than 76%.11 The injury score was obtained by multiplying the degree of damage (0 to 4+) by the percentage of glomeruli showing the corresponding degree of severity.
Real-time polymerase chain reaction
The total RNA (1 μg) of the renal cortex was transcribed into cDNA with superscript III reverse transcriptase and random hexamers (Invitrogen, Carlsbad, CA, USA).10 mRNA was measured by real-time polymerase chain reaction on a LightCycler with software (Roche Diagnostics, Tokyo, Japan) using TaqMan fluorogenic probes. For real-time polymerase chain reaction of p22phox, (TGF)-β, monocyte chemoattractant protein (MCP)-1 and collagen I, all primers and probes were designed by Roche Diagnostics.
Immunohistochemistry
Immunohistochemistry of desmin was performed using a rabbit anti-human desmin antibody (1:70, Dako, Carpinteria, CA, USA).12, 13 Sections were incubated with 3% H2O2 in methanol to inhibit endogenous peroxidase, then incubated with protein-blocking solution (Dako) to block nonspecific antigens. Sections were incubated with an anti-desmin antibody, and later reacted with a labeled streptavidin–biotin peroxidase kit (Dako) and 3-amino-9-ethylcarbazole, which was used for color development. Sections were lightly counterstained with hematoxylin. The glomerular desmin staining was graded from 0 to 4+ according to the percentage of glomerular involvement: 0, no lesions; 1+, less than 25%; 2+, 26 to 50%; 3+, 51 to 75%; 4+, more than 76%.13 The immunostain-positive area was quantified with an image analysis system (model VM-30, Olympus Optical, Tokyo, Japan).
To detect p22phox- and MCP-1-positive cells, we used a goat anti-human p22phox antibody (1:50, Santa Cruz Biotechnology, Santa Cruz, USA) and a goat anti-rat MCP-1 antibody (1:200, Santa Cruz Biotechnology), respectively.10 Sections were incubated with an anti-p22phox antibody, then reacted with a labeled streptavidin–biotin peroxidase kit (Dako) and 3,3-diaminobenzidine. Sections were incubated with an anti-MCP-1 antibody, and later reacted with a labeled streptavidin–biotin peroxidase kit (Dako) and 3-amino-9-ethylcarbazol.
Statistical analysis
Data are expressed as mean±s.e.m. Statistical analyses were performed using a parametric test with Fisher's protected least significant difference. Differences were considered significantly when the P value was <0.05.
Results
Body weight and kidney weight
Body weight, kidney weight and the ratio of kidney weight to body weight 4 weeks after high salt loading are shown in Table 1. Body weight was significantly lighter in the placebo-treated group than in the control group, but there were no significant differences among the active treatment groups. Kidney weight was significantly heavier in the placebo-treated group than in the control group. Kidney weight was significantly lighter only in the irbesartan+efonidipine-treated group compared with that in the placebo-treated group. The ratio of kidney weight to body weight was significantly higher in the placebo-treated group than in the control group, but the ratio was also significantly reduced only in the irbesartan+efonidipine-treated group compared with the placebo-treated group.
Blood pressure, urinary protein excretion and urinary 8-OHdG excretion
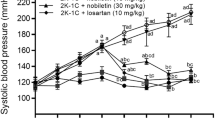
SBP was significantly higher in the placebo-treated group than in the control group at 2 and 4 weeks after high salt loading (Figure 1a). SBP was significantly lower in the irbesartan-, efonidipine- and amlodipine-treated groups than in the placebo-treated group at 2 and 4 weeks after high salt loading (Figure 1a), but there was no significant difference among these three active treatment groups at 2 and 4 weeks. Similar SBP values were observed in the irbesartan+efonidipine-treated group and the irbesartan+amlodipine-treated group at 2 weeks after high salt loading, and the SBP in both these groups was significantly lower than that in the irbesartan-treated group (Figure 1a; P<0.05, irbesartan vs. irbesartan+efonidipine; P<0.01, irbesartan vs. irbesartan+amlodipine). The significant decrease in SBP in the irbesartan+efonidipine-treated group compared with that in the irbesartan-treated group continued at 4 weeks after high salt loading, but the significant difference between the irbesartan- and irbesartan+amlodipine-treated groups disappeared at 4 weeks (Figure 1a).
SBP in the control (open circles, solid line), placebo (closed circles, solid line)-, irbesartan (closed circles, dashed line)-, efonidipine (open triangles, solid line)-, irbesartan+efonidipine (closed triangles, dashed line)-, amlodipine (open squares, solid line)- and irbesartan+amlodipine (closed squares, dashed line)-treated groups at 0, 2 and 4 weeks after high salt loading (a). Urinary protein excretion in the control (open circles, solid line), placebo (closed circles, solid line)-, irbesartan (closed circles, dashed line)-, efonidipine (open triangles, solid line)-, irbesartan+efonidipine (closed triangles, dashed line)-, amlodipine (open squares, solid line)- and irbesartan+amlodipine (closed squares, dashed line)-treated groups at 0, 2 and 4 weeks after high salt loading (b). Urinary 8-OHdG excretion in the control (C), placebo (P)-, irbesartan (I)-, efonidipine (E)-, irbesartan+efonidipine (I+E)-, amlodipine (A)-, and irbesartan+amlodipine (I+A)-treated groups at 4 weeks after high salt loading (c). *P<0.01 and **P<0.01 vs. placebo-treated group. #P<0.05 and ##P<0.01 vs. irbesartan-treated group.
Urinary protein excretion was significantly higher in the placebo-treated group than in the control group at 2 and 4 weeks after high salt loading (Figure 1b). In both the irbesartan- and efonidipine-treated groups, urinary protein excretion was significantly lower than that in the placebo-treated group, but not in the amlodipine-treated group at 4 weeks (Figure 1b). At 2 and 4 weeks after high salt loading, urinary protein excretion was significantly lower in the irbesartan+efonidipine-treated group than in the irbesartan-treated group, but there were no significant differences between the irbesartan- and irbesartan+amlodipine-treated groups (Figure 1b).
Urinary 8-OHdG excretion was significantly higher in the placebo-treated group than in the control group at 4 weeks after high salt loading (Figure 1c). Significant reductions in urinary 8-OHdG excretion in the irbesartan- and efonidipine-treated groups were observed, compared with the placebo-treated group (Figure 1c). In the irbesartan+efonidipine- and irbesartan+amlodipine-treated groups, urinary 8-OHdG excretion was also significantly lower than that in the placebo-treated group (Figure 1c). Furthermore, urinary 8-OHdG excretion was lower in the irbesartan+efonidipine-treated group than in the irbesartan-treated group (Figure 1c).
PRA, serum angiotensin II concentration and plasma aldosterone concentration
Table 2 shows PRA, serum angiotensin II concentrations and plasmaaldosterone concentrations in all groups at 4 weeks after high salt loading. Both PRA and serum angiotensin II concentrations were significantly lower in the placebo-treated group than in the control group. Although PRA tended to be higher in the irbesartan-treated group than in the placebo-treated group, results did not reach statistical significance (P=0.054). Serum angiotensin II concentrations were significantly higher in the irbesartan-treated group than in the placebo-treated group. In the amlodipine- and irbesartan+amlodipine-treated groups, neither PRA nor serum angiotensin II concentrations were significantly different from those in the placebo-treated group. On the other hand, both PRA and serum angiotensin II concentrations were significantly lower in the efonidipine- and irbesartan+efonidipine-treated groups than in the placebo-treated group. Plasma aldosterone concentrations were also significantly lower in the efonidipine- and irbesartan+efonidipine-treated groups than in the placebo-treated group, but no significant differences were seen among the other treatment groups.
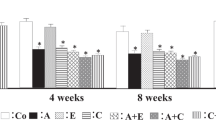
Glomerulosclerosis score
Figure 2a shows typical photographs of glomerular sections with periodic acid Schiff staining. The glomerulosclerosis score was significantly higher in the placebo-treated group than in the control group 4 weeks after high salt loading (Figure 2b). The glomerulosclerosis score was significantly lower in the irbesartan- and efonidipine-treated groups than in the placebo-treated group, but no significant differences in glomerulosclerosis scores were seen between the amlodipine- and placebo-treated groups (Figure 2b). On the other hand, the glomerulosclerosis score was significantly lower in the irbesartan+amlodipine-treated group than in the placebo-treated group (Figure 2b). Further significant decreases in the glomerulosclerosis score were observed in the irbesartan+amlodipine-treated group compared with that in the irbesartan-treated group, but there were no significant differences between the irbesartan- and irbesartan+amlodipine-treated groups (Figure 2b).
Typical photographs of periodic acid Schiff-stained glomuruli in the control, placebo-, irbesartan-, irbesartan+efonidipine- and irbesartan+amlodipine-treated rats (a). Glomerulosclerosis scores in the control (C), placebo (P)-, irbesartan (I)-, efonidipine (E)-, irbesartan+efonidipine (I+E)-, amlodipine (A)- and irbesartan+amlodipine (I+A)-treated groups at 4 weeks after high salt loading (b). *P<0.05 and **P<0.01 vs. placebo-treated group. #P<0.05 vs. irbesartan-treated group.
Desmin staining score
To evaluate the presence of podocyte injury, we performed immunohistochemistry of desmin. Typical photographs of immunostaining for desmin in control, placebo- and irbesartan-, irbesartan+efonidipine- and irbesartan+amlodipine-treated groups are shown in Figure 3a. The desmin staining score was significantly higher in the placebo-treated group than in the control group (Figure 3b). In the irbesartan- and efonidipine-treated groups, but not in the amlodipine-treated group, the desmin staining score was significantly lower than that in the placebo-treated group (Figure 3b). In both the irbesartan+efonidipine- and irbesartan+amlodipine-treated groups, scores were also significantly lower than in the placebo-treated group (Figure 3b). Moreover, the score was significantly lower in the irbesartan+efonidipine-treated group than in the irbesartan-treated group (Figure 3b).
Typical photographs of anti-desmin antibody-immunostained glomeruli in control, placebo-, irbesartan-, irbesartan+efonidipine- and irbesartan+amlodipine-treated rats (a). Desmin staining scores in the control (C), placebo (P)-, irbesartan (I)-, efonidipine (E)-, irbesartan+efonidipine (I+E)-, amlodipine (A)- and irbesartan+amlodipine (I+A)-treated groups 4 weeks after high salt loading (b). **P<0.01 vs. placebo-treated group. #P<0.05 vs. irbesartan-treated group.
Localizations of p22phox and MCP-1, and gene expressions
The p22phox-positive cells were detected in the glomeruli of the placebo-treated rat, but these positive cells were hardly detected in control and irbesartan+efonidipine-treated rats (Figure 4a). On the other hand, MCP-1-positive cells were mainly detected in the tubules in the placebo-treated rat. However, MCP-1-positive cells were slightly detected in control and irbesartan+efonidipine-treated rats (Figure 4b).
Typical photographs of anti-p22phox antibody (a)-immunostained glomeruli and anti-MCP-1 antibody (b)-immunostained renal cortex in control, placebo- and irbesartan+efonidipine-treated rats. Gene expressions of p22phox (c), MCP-1 (d), TGF-β (e) and collagen I (f) in the control (C), placebo (P)-, irbesartan (I)-, efonidipine (E)-, irbesartan+efonidipine (I+E)-, amlodipine (A)- and irbesartan+amlodipine (I+A)-treated groups 4 weeks after high salt loading. *P<0.05 and **P<0.01 vs. placebo-treated group. #P<0.05 vs. irbesartan-treated group.
All gene expressions of p22phox, MCP-1, TGF-β and collagen I in the renal cortex were significantly higher in the placebo-treated group than in the control group (Figure 4). These expressions were significantly lower in the irbesartan-, efonidipine-, irbesartan+efonidipine- and irbesartan+amlodipine-treated groups than in the placebo-treated group (Figure 4). On the other hand, these expressions were not significantly different between the placebo- and amlodipine-treated groups (Figure 4). In the irbesartan+efonidipine-treated group, but not in the irbesartan+amlodipine-treated group, these expressions were significantly lower than in the irbesartan-treated group (Figure 4).
Discussion
Angiotensin II is a potent constrictor of efferent arterioles, and blockade of angiotensin II is useful for the prevention of glomerular injury.14 In the present study of DS rats, SBP was significantly lower in the irbesartan-, efonidipine- and amlodipine-treated groups than in the placebo-treated group at 2 and 4 weeks after high salt loading. Although there were no significant differences among these three groups, the hypotensive effect of irbesartan tended to be weaker than that of efonidipine and amlodipine. On the other hand, both glomerulosclerosis and proteinuria were equally reduced by irbesartan and efonidipine, but not by amlodipine. Angiotensin II levels, as well as angiotensin II type 1 receptor gene expression in the kidney, have been reported to be elevated in the high salt-loaded DS rats used in this study.8 A previous report showed that candesartan cilexetil significantly attenuated glomerulosclerosis and proteinuria along with hypotension in high salt-loaded DS rats.15 In this study, we also observed a significant attenuation of glomerulosclerosis and proteinuria along with hypotension by irbesartan in DS rats at 2 and 4 weeks after high salt loading. Podocytes are localized in the outer aspect of the basement membrane, and injury of these cells is known to be associated with proteinuria.16 Cultured podocytes exposed to mechanical stress enhance angiotensin II type 1 receptor gene expression, which results in podocyte injury.17 In the present study, the desmin staining score, which shows the degree of damaged podocytes, was significantly reduced by irbesartan. These previous observations, along with the findings of our study, show that blockade of angiotensin II is useful for attenuation of glomerulosclerosis and proteinuria in DS rats after high salt loading.
On the other hand, the effect of ARBs might weaken over time. Because angiotensin II is associated with a negative feedback regulation of renin excretion, and ARBs increase PRA, ARBs lead to an attenuated blockade of angiotensin II function.18 Previously, a T- and L-type CCB, mibefradil, has been shown to suppress renin mRNA levels and PRA in normal rats, but an L-type CCB amlodipine did not have this effect.9 In two-kidney, one-clip renal hypertensive rats, a model that is known to show increased PRA levels, mibefradil, significantly reduced PRA; in contrast, amlodipine increased these levels.9 In high salt-loaded DS rats, PRA is known to be suppressed,18 and it was also significantly lowered in the placebo-treated group in our study. However, PRA tended to be higher in the irbesartan-treated group than in the placebo-treated group, although the difference was not significant (P=0.054). On the other hand, a T- and L-type CCB, efonidipine, significantly reduced PRA compared with placebo in high salt-loaded DS rats. Moreover, we observed for the first time that combination treatment with efonidipine and irbesartan significantly reduced PRA compared with monotherapy with irbesartan. However, PRA was not significantly different between amlodipine and placebo, and no significant differences between irbesartan+amlodipine- and irbesartan-treated groups were observed. We also observed that the significant increase in serum angiotensin II concentration by monotherapy with irbesartan was significantly reduced by combination treatment with efonidipine and irbesartan. Therefore, a T-type CCB may be useful to compensate for the weak point of ARBs, which upregulate not only PRA but also serum angiotensin II concentration.
In this study, plasma aldosterone concentration was significantly lowered in DS rats at 4 weeks after high salt loading. High salt loading is known to attenuate renin secretion, angiotensin II concentration and aldosterone concentration in plasma. The plasma aldosterone concentrations in both the efonidipine- and irbesartan+efonidipine-treated groups were significantly lower than that in the placebo-treated group. These findings may be dependent on the reduction of angiotensin II induced by efonidipine. On the other hand, efonidipine may directly reduce aldosterone secretion from the adrenal cortex. Both angiotensin II- and KCl-induced aldosterone secretion levels from human adenocarcinoma cells have been shown to be significantly attenuated by efonidipine, but not by nifedipine.19 In patients with hypertension, efonidipine significantly reduced plasma aldosterone concentrations, whereas amlodipine did not.20, 21 In patients with chronic glomerulonephritis or with hypertension and nephropathy, not only proteinuria but also plasma aldosterone concentrations were significantly lower in those receiving efonidipine compared with those receiving amlodipine.22, 23 In a rat model of renal ablation, attenuation of aldosterone function reduced podocyte injury.24 In high salt-loaded DS rats, although hydralazine and eplerenone equally reduced SBP in high salt-loaded DS rats, eplerenone, but not hydralazine, significantly reduced glomerulosclerosis, proteinuria and desmin staining score, an index of podocyte injury.13 Eplerenone also reduced p22phox, MCP-1 and TGF-β gene expressions, but hydralazine did not.13 Although plasma aldosterone concentration might be lowered under high salt loading, the importance of eplerenone showing renal protection may suggest the dissociation between the plasma aldosterone concentration and renal lesion in high salt-loaded DS rats. In contrast, chronic infusion of aldosterone with high salt loading augmented glomerulosclerosis, proteinuria and desmin expression in uninephrectomized rats.25 However, it is unclear which is more important for the renal protection between plasma aldosterone concentration and renal lesion, and further studies are needed. In this study, we observed that efonidipine significantly reduced glomerulosclerosis, proteinuria and the desmin staining score, but amlodipine did not. Moreover, efonidipine, similar to eplerenone, significantly reduced p22phox, MCP-1, TGF-β and collagen I gene expressions. MCP-1 stimulates collagen synthesis by upregulation of TGF-β expression.26 N-acetyl-cysteine, a reactive oxygen species scavenger, significantly reduced MCP-1 expressions and showed a significant attenuation of glomerulosclerosis and proteinuria.27 Therefore, the mechanism by which efonidipine attenuates glomerulosclerosis and proteinuria might be partially explained by its ability to reduce aldosterone-induced functions.
SBP was almost the same between the efonidipine and amlodipine groups at 2 and 4 weeks in this study. Significant attenuation of glomerulosclerosis and proteinuria was seen in the efonidipine-treated group compared with the placebo-treated group; these attenuations were not seen in the amlodipine-treated group. Such differences between efonidipine and amlodipine regarding attenuation of glomerulosclerosis and proteinuria might be based on the different effects of these agents on afferent and efferent arterioles. Efonidipine is known to dilate both afferent and efferent arterioles, but amlodipine predominantly dilates only afferent arterioles.4, 5, 6 The reason for the different function between these CCBs may be the different distribution. α1C L-type calcium channel subunits mainly exist in afferent arterioles, and α1H T-type calcium channel subunits exist in both afferent and efferent arterioles.28 Previously, amlodipine resulted in a greater increase in the glomerular filtration rate than in renal plasma flow under angiotensin II-induced vasoconstriction in rats; in contrast, efonidipine potently increased renal plasma flow more than the glomerular filtration rate.29, 30 Other T- and L-type CCBs have also been reported to dilate both afferent and efferent arterioles in rat models.31, 32 In this study, efonidipine, but not amlodipine, significantly reduced multiple components of the renin–angiotensin–aldosterone system. For example, both angiotensin II and aldosterone are potent constrictors of efferent arterioles, and these contractions are attenuated by T-type CCBs, but not L-type CCBs.33, 34 Therefore, not only direct dilation of efferent arterioles but also indirect dilation by reduction of angiotensin II and aldosterone might be important for the attenuation of glomerulosclerosis and proteinuria by T-type CCBs.
We also evaluated two combination therapies with different types of CCBs: irbesartan+efonidipine and irbesartan+amlodipine. Significant SBP-lowering effects of irbesartan+efonidipine and irbesartan+amlodipine, compared with irbesartan alone, were observed at 2 weeks after high salt loading, with no significant differences between the two combination groups. However, the significant SBP-lowering effect continued to be observed in the irbesartan+efonidipine-treated group after 4 weeks of high salt loading, but disappeared in the irbesartan+amlodipine-treated group. An NADPH oxidase subunit p22phox gene expression was significantly attenuated by combination therapy with irbesartan and efonidipine compared with irbesartan monotherapy at 4 weeks, whereas there was no significant difference between combination therapy with irbesartan and amlodipine and irbesartan monotherapy. In high salt-loaded DS rats, a superoxide dismutase mimetic, tempol, was shown to significantly reduce p22phox gene expression in glomeruli, proteinuria and SBP.35 In the same rat model, an NADPH inhibitor, apocynin, also showed significant attenuation of proteinuria, as well as a significant reduction of SBP.36 In this study, the significant reduction of p22phox by combination therapy with irbesartan and efonidipine compared with irbesartan monotherapy may have contributed to the significant hypotensive effects seen with combination therapy compared with irbesartan alone.
In cultured proximal tubular cells and ischemia–reperfusion-induced tubular damage, an L-type CCB, azelnidipine, attenuated mitochondrial injury, and ATP depletion-induced apoptosis in hypoxic renal tubular cells.37 In a recent paper, amlodipine improved oxygen conditions, restored the loss of peritubular capillaries and prevented tubulointerstitial fibrosis in the kidney of DS rats with high salt loading for 10 weeks.38 However, we did not observe any tubulointerstitial fibrosis in the kidneys of high salt-loaded DS rats at 4 weeks. The usefulness of combination therapy with irbesartan and amlodipine for protection of tubules and the interstitium should be evaluated in further studies.
In conclusion, both irbesartan and efonidipine were useful for attenuating glomerulosclerosis and proteinuria. The combination of irbesartan and efonidipine was more effective at attenuating glomerulosclerosis and proteinuria than was monotherapy with irbesartan. The attenuating effect of efonidipine on the renin–angiotensin–aldosterone system may have an important role in the beneficial effect of combination therapy.
References
Anderson PW, Zhang XY, Tian J, Correale JD, Xi XP, Yang D et al. Insulin and angiotensin II are additive in stimulating TGF-β1 and matrix mRNAs in mesangial cells. Kidney Int 1996; 50: 745–753.
Pelayo JC, Quan AH, Shanley PF . Angiotensin II control of the renal microcirculation in rats with reduced renal mass. Am J Physiol 1990; 258: F414–F422.
Hayashi K, Wakino S, Sugano N, Ozawa Y, Homma K, Saruta T . Ca2+ channel subtypes and pharmacology in the kidney. Circ Res 2007; 100: 342–353.
Brenner BM . Nephron adaptation to renal injury or ablation. Am J Physiol 1985; 249: F324–F337.
Hayashi K, Nagahama T, Oka K, Epstein M, Saruta T . Disparate effects of calcium antagonists on renal microcirculation. Hypertens Res 1996; 19: 31–36.
Honda M, Hayashi K, Matsuda H, Kubota E, Tokuyama H, Okubo K et al. Divergent renal vasodilator action of L- and T-type calcium antagonists in vivo. J Hypertens 2001; 19: 2031–2037.
Fujiwara K, Kanno Y, Hayashi K, Takenaka T, Saruta T . Renal protective effects of efonidipine in partially nephrectomized spontaneously hypertensive rats. Clin Exp Hypertens 1998; 20: 295–312.
Kobori H, Nishiyama A, Abe Y, Navar LG . Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension 2003; 41: 592–597.
Wagner C, Krämer BK, Hinder M, Kieninger M, Kurtz A . T-type and L-type calcium channel blockers exert opposite effects on renin secretion and renin gene expression in conscious rats. Br J Pharmacol 1998; 124: 579–585.
Takai S, Jin D, Ikeda H, Sakonjo H, Miyazaki M . Significance of angiotensin II receptor blockers with high affinity to angiotensin II type 1 receptors for vascular protection in rats. Hypertens Res 2009; 32: 853–860.
Uehara Y, Hirawa N, Takeda T, Numabe A, Kawabata Y, Nagoshi H et al. Possible linkage between renal injury and cardiac remodeling in Dahl salt-sensitive rats treated with the calcium channel antagonist benidipine. Hypertens Res 1995; 18: 245–253.
Takai S, Kirimura K, Jin D, Muramatsu M, Yoshikawa K, Mino Y et al. Significance of angiotensin II receptor blocker lipophilicities and their protective effect against vascular remodeling. Hypertens Res 2005; 28: 593–600.
Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T . Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension 2006; 47: 1084–1093.
Remuzzi G, Perico N, Macia M, Ruggenenti P . The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int 2005; 99: S57–S65.
Nakaya H, Sasamura H, Mifune M, Shimizu-Hirota R, Kuroda M, Hayashi M et al. Prepubertal treatment with angiotensin receptor blocker causes partial attenuation of hypertension and renal damage in adult Dahl salt-sensitive rats. Nephron 2002; 91: 710–718.
Pavenstädt H, Kriz W, Kretzler M . Cell biology of the glomerular podocyte. Physiol Rev 2003; 83: 253–307.
Durvasula RV, Petermann AT, Hiromura K, Blonski M, Pippin J, Mundel P et al. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int 2004; 65: 30–39.
von Lutterotti N, Camargo MJ, Campbell Jr WG, Mueller FB, Timmermans PB, Sealey JE et al. Angiotensin II receptor antagonist delays renal damage and stroke in salt-loaded Dahl salt-sensitive rats. J Hypertens 1992; 10: 949–957.
Imagawa K, Okayama S, Takaoka M, Kawata H, Naya N, Nakajima T et al. Inhibitory effect of efonidipine on aldosterone synthesis and secretion in human adrenocarcinoma (H295R) cells. J Cardiovasc Pharmacol 2006; 47: 133–138.
Tanaka T, Tsutamoto T, Sakai H, Fujii M, Yamamoto T, Horie M . Comparison of the effects of efonidipine and amlodipine on aldosterone in patients with hypertension. Hypertens Res 2007; 30: 691–697.
Tsutamoto T, Tanaka T, Nishiyama K, Yamaji M, Kawahara C, Fujii M et al. Long-term effect of efonidipine therapy on plasma aldosterone and left ventricular mass index in patients with essential hypertension. Hypertens Res 2009; 32: 670–674.
Ishimitsu T, Kameda T, Akashiba A, Takahashi T, Ohta S, Yoshii M et al. Efonidipine reduces proteinuria and plasma aldosterone in patients with chronic glomerulonephritis. Hypertens Res 2007; 30: 621–626.
Sasaki H, Saiki A, Endo K, Ban N, Yamaguchi T, Kawana H et al. Protective effects of efonidipine, a T- and L-type calcium channel blocker, on renal function and arterial stiffness in type 2 diabetic patients with hypertension and nephropathy. J Atheroscler Thromb 2009; 16: 568–575.
Aldigier JC, Kanjanbuch T, Ma LJ, Brown NJ, Fogo AB . Regression of existing glomerulosclerosis by inhibition of aldosterone. J Am Soc Nephrol 2005; 16: 3306–3314.
Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T . Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension 2007; 49: 355–364.
Gharaee-Kermani M, Denholm EM, Phan SH . Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem 1996; 271: 17779–17784.
Fujii S, Zhang L, Kosaka H . Albuminuria, expression of nicotinamide adenine dinucleotide phosphate oxidase and monocyte chemoattractant protein-1 in the renal tubules of hypertensive Dahl salt-sensitive rats. Hypertens Res 2007; 30: 991–998.
Hansen PB, Jensen BL, Andreasen D, Skøtt O . Differential expression of T- and L-type voltage-dependent calcium channels in renal resistance vessels. Circ Res 2001; 89: 630–638.
Loutzenhiser RD, Epstein M, Fischetti F, Horton C . Effects of amlodipine on renal hemodynamics. Am J Cardiol 1989; 64: 122I–127I.
Ozawa Y, Hayashi K, Nagahama T, Fujiwara K, Saruta T . Effect of T-type selective calcium antagonist on renal microcirculation. Hypertension 2001; 38: 343–347.
Nakamura Y, Ono H, Frohlich ED . Differential effects of T- and L-type calcium antagonists on glomerular dynamics in spontaneously hypertensive rats. Hypertension 1999; 34: 273–278.
Masumiya H, Tanaka Y, Tanaka H, Shigenobu K . Inhibition of T-type and L-type Ca2+ currents by aranidipine, a novel dihydropyridine Ca2+ antagonist. Pharmacology 2000; 61: 57–61.
Arima S, Kohagura K, Xu HL, Sugawara A, Abe T, Satoh F et al. Nongenomic vascular action of aldosterone in the glomerular microcirculation. J Am Soc Nephrol 2003; 14: 2255–2263.
Saruta T, Kanno Y, Hayashi K, Konishi K . Antihypertensive agents and renal protection: calcium channel blockers. Kidney Int 1996; 55 (Suppl): S52–S56.
Nishiyama A, Yoshizumi M, Hitomi H, Kagami S, Kondo S, Miyatake A et al. The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. J Am Soc Nephrol 2004; 15: 306–315.
Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS et al. NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 2008; 295: R1858–R1865.
Tanaka T, Nangaku M, Miyata T, Inagi R, Ohse T, Ingelfinger JR et al. Blockade of calcium influx through L-type calcium channels attenuates mitochondrial injury and apoptosis in hypoxic renal tubular cells. J Am Soc Nephrol 2004; 15: 2320–2333.
Du J, Fan YY, Hitomi H, Kiyomoto H, Kimura S, Kong CZ et al. Mineralocorticoid receptor blockade and calcium channel blockade have different renoprotective effects on glomerular and interstitial injury in rats. Am J Physiol Renal Physiol 2009; 297: F802–F808.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takai, S., Jin, D., Sakonjo, H. et al. Combination therapy with irbesartan and efonidipine for attenuation of proteinuria in Dahl salt-sensitive rats. Hypertens Res 33, 953–959 (2010). https://doi.org/10.1038/hr.2010.90
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2010.90
Keywords
This article is cited by
-
Transplantation of mesenchymal stem cells into the renal medulla attenuated salt-sensitive hypertension in Dahl S rat
Journal of Molecular Medicine (2014)
-
Powerful vascular protection by combining cilnidipine with valsartan in stroke-prone, spontaneously hypertensive rats
Hypertension Research (2013)