Abstract
There is growing recognition of cardiovascular consequences of obstructive sleep apnea (OSA). Recurrent episodes of airway obstructions result in hypoxia and hypercapnia increasing sympathetic neural tone, which in turn causes vasoconstriction and marked increases in blood pressure (BP). BP response to OSA may be important in understanding the absence of nocturnal BP fall in the subgroup of hypertensive patients termed ‘non-dippers’. Even mild sleep apnea can increase nocturnal BP through different mechanisms including hypoxemia, sympathetic activation, mechanical changes and disruption of normal sleep. Sleep apnea may be an important factor in determining the increased cardiovascular risk in hypertensive non-dippers. Effective treatment of sleep apnea may attenuate neurohumoral and metabolic abnormalities, improve diurnal BP control and conceivably reduce cardiovascular risk. This review examines the evidence linking OSA to non-dipping pattern of hypertension, and discusses potential mechanisms underlying this link. We will review first, prognostic value of nighttime BP; second, the cardiovascular consequences of sleep apnea; third, the evidence for altered diurnal BP profile in sleep apnea; fourth, the mechanisms contributing to both nocturnal and daytime hypertension in sleep apnea; fifth, the benefits of sleep apnea treatment and finally implications for hypertension management.
Similar content being viewed by others
Introduction
Ambulatory monitoring has shown that the 24-h blood pressure (BP) profile is characterized by considerable variability and a marked diurnal rhythm.1, 2, 3, 4, 5 A substantial component of BP variability during ambulatory monitoring can be accounted for by changes in activity. However, the physiological mechanisms responsible for the variability and the diurnal rhythm of BP are not completely understood.6 Nevertheless, there is growing evidence that sympathetic nerve activity contributes importantly to BP variability and the diurnal BP profile.7, 8
Normally, BP decreases during sleep by at least 10% of the awake value, and occurrence of ‘dipping’ correlates directly with the amount of deep sleep and inversely with indices of sleep fragmentation.9 There has been great interest in the mechanisms and clinical significance of the ‘dipper’ or ‘non-dipper’ pattern of ambulatory BP profiles.
Obstructive sleep apnea (OSA) and the consequent nighttime BP surges may be involved in the increased cardiovascular morbidity that characterizes those hypertensive patients without nocturnal BP decline.
This review examines the evidence linking OSA to non-dipping pattern of hypertension, and discusses potential mechanisms underlying this link. We will review first, prognostic value of nighttime BP; second, the cardiovascular consequences of OSA; third, the evidence for altered diurnal BP profile in OSA; fourth, the mechanisms contributing to both nocturnal and daytime hypertension in OSA; fifth, the benefits of OSA treatment and finally implications for hypertension management. Because of the space limitations, only few references relevant to specific areas can be included.
Prognostic value of nighttime BP
Ambulatory BP is a better predictor of cardiovascular risk than office BP. The risk of cardiovascular morbidity and mortality increases more steeply from office to home, day, 24 h and night BP10, 11 (Figure 1).
Increase in 11-year risk of cardiovascular (CV) mortality for 10 mm Hg increase in office, home and ambulatory systolic blood pressure (SBP) and diastolic blood pressure (DBP) at various initial values in the Pressioni Arteriose Monitorate E Loro Associazioni study (from Sega et al.,11 with permission).
A blunted nocturnal BP dip phenomenon is common in hypertensive patients.12 It has been suggested that both non-dipping13, 14, 15 and extreme dipping16, 17, 18 are associated with more pronounced target organ damage. Furthermore, a lack of nocturnal dipping has been related to an increased risk of cardiovascular events.19, 20, 21, 22, 23 The night-to-day blood pressure ratio predicts cardiovascular and non-cardiovascular mortality.24 Finally, a recent study by Muxfeldt et al.25 in resistant hypertension indicates that the nocturnal BP variability patterns provide valuable prognostic information for stratification of cardiovascular morbidity and mortality risk, above and beyond other traditional cardiovascular risk factors and mean ambulatory BP levels.
Interestingly, a recent study by Eguchi et al.26 indicates that also nocturnal non-dipping of heart rate predicts cardiovascular events in hypertensive patients. The risk of future cardiovascular events was shown to be markedly higher in those whose heart rate does not exhibit the typical nocturnal decline (Figure 2). This relationship was independent of non-dipping of BP.
Event-free survival in hypertensive patients according to heart rate dipping pattern in 497 hypertensive patients (from Eguchi et al.,26 with permission).
Cardiovascular consequences of OSA
There is growing recognition of cardiovascular consequences of OSA.27, 28, 29 Several studies provided evidence that OSA patients have increased BP and a higher incidence of hypertension.30, 31, 32 Conversely, patients with essential hypertension are more likely to have sleep-disordered breathing. This association is particularly evident within a group of patients with hypertension refractory to conventional therapy. Up to 84% of these hypertensive subjects may have previously undiagnosed OSA.33, 34
The Wisconsin Sleep Cohort Study has shown a dose–response relationship of sleep-disordered breathing at baseline and the incidence of hypertension in a 4 year observation.35 The association between OSA and arterial hypertension is affected by aging. The analysis of the Sleep Heart Health Study36 has shown an independent association of OSA and hypertension in the group of middle-aged, but not in the elderly subjects. Noda et al.37 have shown that the survival rate in middle-aged patients with OSA was significantly lower than that in the age and sex-adjusted control Japanese population, but this pattern was not seen among the elderly patients. Distinct effects of OSA in populations of different ages have been attributed to differences in concomitant disease, underlying risk factors for sleep disordered breathing (similar to relative contributions of obesity vs. ventilatory control abnormalities) and to differences in physiological responses to intermittent upper airway occlusion.36
OSA has also been shown to be an independent risk factor for the development of left ventricular hypertrophy.38, 39 Several longitudinal studies have documented increased cardiovascular morbidity in OSA patients.40, 41, 42 OSA has been shown to be a significant risk factor for the composite outcome of death and stroke, independent of other risk factors including hypertension and atrial fibrillation.43 Finally, patients with OSA have a peak in sudden death from cardiac causes during the sleeping hours, which contrasts strikingly with the findings in the general population.44
OSA and non-dippers
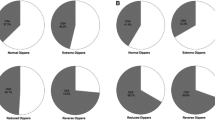
Prevalence of hypertension is underdiagnosed in OSA patients if BP is assessed by office readings only. In the Wisconsin Sleep Cohort Study, Young et al.31 found a linear relationship between 24-h BP and AHI that was independent of confounding factors such as body mass index. Baguet et al.45 have shown that ambulatory BP monitoring might be of particular significance in the hypertension diagnosis of OSA patients (Figure 3). Although 42% of their OSA patients showed office hypertension, 58% had daytime hypertension and 76% had nighttime hypertension. Hypertensive patients, in whom there is an absence of a nocturnal BP decline (non-dippers), are at increased risk for cardiac and vascular events.46, 47 The nocturnal BP profile in non-dipper hypertensive patients is strikingly similar to that described in studies of 24 h BP measurements in patients with OSA. Pankow et al.48 have shown that non-dipping of nocturnal BP in patients with OSA is related to apnea severity. Lavie et al.49 have also reported that BP during sleep correlated significantly with the apnea hypopnea index. In a study, directed specifically at male non-dippers with essential hypertension, Portaluppi et al.50 concluded that hypertensive non-dippers had a high probability of coexisting sleep-disordered breathing. Interestingly, a significant relationship between evening-to-morning BP difference and AHI was confirmed in men but not in women.51 A ‘non-dipping’ pattern was found in 48–84% of patients with OSA, and its frequency increases with OSA severity.52, 53
Relative proportions of different pattern of hypertension (HT) using clinical and ambulatory blood pressure measurements (ABPM) in newly diagnosed obstructive sleep apnea patients. BP, blood pressure; NT, normotension (from Baguet et al.,45 with permission).
Detection of intermittent BP surges during sleep and non-dipping pattern in OSA syndrome patients can be further improved by the newly developed non-invasive hypoxia-triggered monitoring system.54, 55
Mechanisms contributing to non-dipping pattern of hypertension in OSA
Recurrent episodes of airway obstructions result in hypoxia and hypercapnia increasing sympathetic neural tone, which in turn causes vasoconstriction.56 Sympathetic nerve activity rises progressively during the time of apnea and is enhanced further by the arousal. On resumption of breathing, cardiac output increases which coincides with the constricted peripheral vasculature. This results in marked increases in arterial pressure. BP and sympathetic activity in sleep-apneic patients are highest during rapid eye movement sleep, as it is during this sleep stage that apneas are most prolonged. Because of repetitive vasoconstriction and BP surges, BP overall does not decrease during sleep in patients with OSA.
OSA-related repetitive hypoxemia with consequent chemoreflex activation, sympathetic excitation and nighttime BP surges may be involved in the above described cardiovascular events in sleep-apneic patients (Figure 4). This hemodynamic instability, related to respiratory efforts, hypoxemia and arousal, may lead to increased risk of cardiac arrhythmias and sudden cardiac death. It may also favor ischemic or hemorrhagic stroke, decompensation of a chronic heart failure condition, as well as the development of chronic arterial hypertension in these patients.
High levels of sympathetic activity in OSA patients are associated with profound abnormalities in cardiovascular variability during wakefulness. This alteration occurs even in the absence of hypertension or heart failure. OSA patients' BP variability exhibit marked increase, heart rate is faster and the RR variability is decreased.57 The degree of derangement in cardiovascular variability is closely linked to the severity of syndrome. Possible mechanisms underlying autonomic control abnormalities include chemoreflex dysfunction58 and impaired baroreceptor function.59, 60
Other factors implicated in the non-dipping pattern of hypertension in OSA patients include impaired endothelium-dependent vasodilation,61, 62 suppressed nitric oxide production,63, 64 higher levels of plasma asymmetric dimethylarginine (ADMA),65 oxidative stress,66, 67 increased concentrations of endothelin-1,68 low-grade inflammation,69, 70, 71 and increased levels of circulating intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and L-selectin.72
Benefits of OSA treatment
The most common and highly efficient therapeutic procedure of eliminating airway obstruction at night consists of use of a continuous positive airway pressure (CPAP). In addition to the use of airway assistance device, OSA therapeutic strategy comprises weight loss (inclusive of bariatric surgery), upper airway surgical procedures, use of mandibular protruding devices, avoidance of alcohol and sedative hypnotics and sleep postural changes. In case of patients with concomitant heart failure, treatment options include bilevel and adaptive pressure support servoventilation.
Treatment with CPAP results in acute and marked reduction in nocturnal sympathetic nerve traffic and blunts BP surges during sleep. Long-term effective CPAP treatment has been shown to improve nocturnal and daytime BP control.73, 74, 75 The beneficial effects of the use of a CPAP appliance is especially evident in patients with more severe OSA.76
CPAP therapy leads to beneficial neurohormonal changes that may facilitate hypertension management. Long-term CPAP treatment decreases neural sympathetic activity measured by microneurography in otherwise healthy OSA patients.77 There is evidence that CPAP therapy may reduce risk of incidence of fatal and non-fatal cardiovascular events in male OSA patients as compared with untreated OSA patients.78
Implications for hypertension management
Close link between OSA and hypertension has important implications for cardiovascular prevention and treatment. OSA may contribute to elevated levels of BP in a large proportion of hypertensives, and should be strongly suspected in obese individuals with resistant hypertension, especially in those with non-dipping pattern of BP. Already in 1997, the 6th Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure had recommended OSA to be considered in patients with resistant hypertension.79 The more recent seventh report from this Committee has broadened their recommendations. According to the Seventh Joint National Committee Report, all of the hypertensive patients with body mass index above 27 kg m−2 should be thoroughly screened for the presence of sleep-disordered breathing.80 The 2007 European Society of Hypertension and of the European Society of Cardiology guidelines have also listed OSA as one of the most important causes of resistant hypertension.81
Conclusions
BP response to sleep in OSA patients may be important in understanding the absence of nocturnal BP decrease in the subgroup of hypertensive patients termed ‘non-dippers’. Even mild OSA can increase nocturnal BP through different mechanisms including hypoxemia, sympathetic activation, mechanical changes and disruption of normal sleep. OSA can prevent the physiological decrease in BP and, when severe, it can increase nocturnal BP compared with awake values. Moreover, OSA increases BP variability during sleep, with further potentiation of chronic stress on the vessel wall. OSA may be an important factor in determining the increased cardiovascular risk in hypertensive non-dippers. Effective treatment of OSA may attenuate neurohumoral and metabolic abnormalities, improve BP control and conceivably reduce cardiovascular risk.
References
O'Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redon J, Staessen J, Stergiou G, Verdecchia P, European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens 2003; 21: 821–848.
Mancia G, Di Rienzo M, Parati G . Ambulatory blood pressure monitoring use in hypertension research and clinical practice. Hypertension 1993; 21: 510–524.
Pickering TG, James GD . Determinants and consequences of the diurnal rhythm of blood pressure. Am J Hypertens 1993; 6: 166S–169S.
Staessen J, Bulpitt CJ, O'Brien E, Cox J, Fagard R, Stanton A, Thijs L, Van Hulle S, Vyncke G, Amery A . The diurnal blood pressure profile: a population study. Am J Hypertens 1992; 5: 386–392.
Parati G, Valentini M . Do we need out-of-office blood pressure in every patient? Curr Opin Cardiol 2007; 22: 321–328.
Pickering TG, Shimbo D, Haas D . Ambulatory blood-pressure monitoring. N Engl J Med 2006; 354: 2368–2374.
Narkiewicz K, Winnicki M, Schroeder K, Phillips BG, Kato M, Cwalina E, Somers VK . Relationship between muscle sympathetic nerve activity and diurnal blood pressure profile. Hypertension 2002; 39: 168–172.
Grassi G, Bombelli M, Seravalle G, Dell'oro R, Quarti-Trevano F . Diurnal blood pressure variation and sympathetic activity. Hypertens Res 2010; 33: 381–385.
Loredo JS, Nelesen R, Ancoli-Israel S, Dimsdale JE . Sleep quality and blood pressure dipping in normal adults. Sleep 2004; 27: 1097–1103.
Staessen JA, Thijs L, Fagard R, O'Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J . Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA 1999; 282: 539–546.
Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G . Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation 2005; 111: 1777–1783.
de la Sierra A, Redon J, Banegas JR, Segura J, Parati G, Gorostidi M, de la Cruz JJ, Sobrino J, Llisterri JL, Alonso J, Vinyoles E, Pallarés V, Sarría A, Aranda P, Ruilope LM, Spanish Society of Hypertension Ambulatory Blood Pressure Monitoring Registry Investigators. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension 2009; 53: 466–472.
Palatini P, Penzo M, Racioppa A, Zugno E, Guzzardi G, Anaclerio M, Pessina AC . Clinical relevance of nighttime blood pressure and of daytime blood pressure variability. Arch Intern Med 1992; 152: 1855–1860.
Verdecchia P, Gatteschi C, Benemio G, Boldrini F, Guerreri M, Porcellati C . Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation 1990; 81: 528–536.
Verdecchia P, Schillaci G, Gatteschi C, Zampi I, Battistelli M, Bartoccini C, Porcellati C . Blunted nocturnal fall in blood pressure in hypertensive women with future cardiovascular morbid events. Circulation 1993; 88: 986–992.
Kario K, Matsuo T, Kobayashi H, Imiya M, Matsuo M, Shimada K . Relation between nocturnal fall of blood pressure and silent cerbrovascular damage in elderly hypertensives: advanced silent cerebrovascular damage in extreme-dippers. Hypertension 1996; 27: 130–135.
Pierdomenico SD, Bucci A, Constanini F, Lapena Cuccurullo F, Mezzetti A . Circadian blood pressure changes and myocardial ischemia in hypertensive patients with coronary artery disease. J Am Coll Cardiol 1998; 31: 1627–1634.
Kario K, Shimada K, Pickering TG . Abnormal nocturnal blood pressure falls in elderly hypertension: clinical significance and determinants. J Cardiovasc Pharmacol 2003; 41 (Suppl 1): S61–S66.
Parati G, Valentini M . Prognostic relevance of blood pressure variability. Hypertension 2006; 47: 137–138.
Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y . Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002; 20: 2183–2189.
Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K . Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 2000; 38: 852–857.
Metoki H, Ohkubo T, Kikuya M, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y . Prognostic significance for stroke of a morning pressure surge and a nocturnal blood pressure decline: the Ohasama study. Hypertension 2006; 47: 149–154.
Eguchi K, Ishikawa J, Hoshide S, Pickering TG, Schwartz JE, Shimada K, Kario K . Night time blood pressure variability is a strong predictor for cardiovascular events in patients with type 2 diabetes. Am J Hypertens 2009; 22: 46–51.
Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Kuznetsova T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Wang J, Sandoya E, O'Brien E, Staessen JA, International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) investigators. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370: 1219–1229.
Muxfeldt ES, Cardoso CR, Salles GF . Prognostic value of nocturnal blood pressure reduction in resistant hypertension. Arch Intern Med 2009; 169: 874–880.
Eguchi K, Hoshide S, Ishikawa J, Pickering TG, Schwartz JE, Shimada K, Kario K . Nocturnal nondipping of heart rate predicts cardiovascular events in hypertensive patients. J Hypertens 2009; 27: 2265–2270.
Bradley TD, Floras JS . Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009; 373: 82–93.
Parati G, Lombardi C, Narkiewicz K . Sleep apnea: epidemiology, pathophysiology, and relation to cardiovascular risk. Am J Physiol Regul Integr Comp Physiol 2007; 293: R1671–R1683.
Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T, Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008; 118: 1080–1111.
Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Leiby BE, Vela-Bueno A, Kales A . Association of hypertension and sleep-disordered breathing. Arch of Intern Med 2000; 160: 2289–2295.
Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, Skatrud J . Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med 1997; 157: 1746–1752.
Nieto FJ, Young TB, Bonnie KL, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz M, Pickering TC, for the Sleep Heart Health Study. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 2000; 283: 1829–1836.
Isaksson A, Svanborg E . Obstructive sleep apnea syndrome in male hypertensives, refractory to drug therapy. Nocturnal automatic blood pressure measurements—an aid to diagnosis? Clin Exp Hypertens A 1991; 13: 1195–1212.
Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, Leung RS, Bradley TD . High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19: 2271–2277.
Peppard PE, Young T, Palta M, Skatrud J . Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342: 1378–1784.
Haas DC, Foster GL, Nieto FJ, Redline S, Resnick HE, Robbins JA, Young T, Pickering TG . Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation 2005; 111: 614–621.
Noda A, Okada T, Yasuma F, Sobue T, Nakashima N, Yokota M . Prognosis of the middle-aged and aged patients with obstructive sleep apnea syndrome. Psychiatry Clin Neurosci 1998; 52: 79–85.
Kraiczi H, Peker Y, Caidahl K, Samuelsson A, Hedner J . Blood pressure, cardiac structure and severity of obstructive sleep apnea in a sleep clinic population. J Hypertens 2001; 19: 2071–2078.
Tanriverdi H, Evrengul H, Kaftan A, Kara CO, Kuru O, Tanriverdi S, Ozkurt S, Semiz E . Effect of obstructive sleep apnea on aortic elastic parameters—relationship to left ventricular mass and function. Circ J 2006; 70: 737–743.
Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J . Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med 2002; 166: 159–165.
Marin JM, Carrizo SJ, Vicente E, Agusti AG . Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365: 1046–1053.
Doherty LS, Kiely JL, Swan V, McNicholas WT . Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest 2005; 127: 2076–2084.
Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V . Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005; 353: 2034–2041.
Gami AS, Howard DE, Olson EJ, Somers VK . Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352: 1206–1214.
Baguet JP, Hammer L, Levy P, Pierre H, Rossini E, Mouret S, Ormezzano O, Mallion JM, Pepin JL . Night-time and diastolic hypertension are common and underestimated conditions in newly diagnosed apnoeic patients. J Hypertens 2005; 23: 521–527.
Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, Porcellati C . Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation 1990; 81: 528–536.
Verdecchia P, Schillaci G, Gatteschi C, Zampi I, Battistelli M, Bartoccini C, Porcellati C . Blunted nocturnal fall in blood pressure in hypertensive women with future cardiovascular morbid events. Circulation 1993; 88: 986–992.
Pankow W, Nabe B, Lies A, Becker H, Kohler U, Kohn F-V, Lohmann FW . Influence of sleep apnea on 24-h blood pressure. Chest 1997; 112: 1253–1258.
Lavie P, Yoffe N, Berger I, Peled R . The relationship between the severity of sleep apnea syndrome and 24-h blood pressure values in patients with obstructive sleep apnea. Chest 1993; 103: 717–7721.
Portaluppi F, Provini F, Cortelli P, Plazzi G, Bertozzi N, Manfredini R, Fersini C, Lugaresi E . Undiagnosed sleep-disordered breathing among male nondippers with essential hypertension. J Hypertens 1997; 15: 1227–1233.
Sforza E, Lugaresi E . Determinants of the awakening rise in systemic blood pressure in obstructive sleep apnea syndrome. Blood Press 1995; 4: 218–225.
Nabe B, Lies A, Pankow W, Kohl FV, Lohmann FW . Determinants of circadian blood pressure rhythm and blood pressure variability in obstructive sleep apnoea. J Sleep Res 1995; 4 (S1): 97–101.
Suzuki M, Guilleminault C, Otsuka K, Shiomi T . Blood pressure ‘dipping’ and ‘non-dipping’ in obstructive sleep apnea syndrome patients. Sleep 1996; 19: 382–387.
Shirasaki O, Yamashita S, Kawara S, Tagami K, Ishikawa J, Shimada K, Kario K . A new technique for detecting sleep apnea-related ‘midnight’ surge of blood pressure. Hypertens Res 2006; 29: 695–702.
Kario K . Obstructive sleep apnea syndrome and hypertension: ambulatory blood pressure. Hypertens Res 2009; 32: 428–432.
Somers VK, Dyken ME, Clary MP, Abboud FM . Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96: 1897–1904.
Narkiewicz K, Montano N, Cogliati C, van de Borne PJH, Dyken ME, Somers VK . Altered cardiovascular variability in obstructive sleep apnea. Circulation 1998; 98: 1071–1077.
Narkiewicz K, van de Borne PJH, Montano N, Dyken M, Phillips BG, Somers VK . The contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation 1998; 97: 943–945.
Parati G, Di Rienzo M, Bonsignore MR, Insalaco G, Marrone O, Castiglioni P, Bonsignore G, Mancia G . Autonomic cardiac regulation in obstructive sleep apnea syndrome: evidence from spontaneous baroreflex analysis during sleep. J Hypertens 1997; 15: 1621–1626.
Narkiewicz K, Pesek CA, Kato M, Phillips BG, Davison DE, Somers VK . Baroreflex control of sympathetic activity and heart rate in obstructive sleep apnea. Hypertension 1998; 32: 1039–1043.
Kraiczi H, Caidahl K, Samuelsson A, Peker Y, Hedner J . Impairment of vascular endothelial function and left ventricular filling. Association with the severity of apnea-induced hypoxemia during sleep. Chest 2001; 119: 1085–1091.
Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK . Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation 2000; 102: 2607–2610.
Ip MS, Lam B, Chan LY, Zheng L, Tsang KW, Fung PC, Lam WK . Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med 2000; 162: 2166–2171.
Noda A, Nakata S, Koike Y, Miyata S, Kitaichi K, Nishizawa T, Nagata K, Yasuma F, Murohara T, Yokota M . Continuous positive airway pressure improves daytime baroreflex sensitivity and nitric oxide production in patients with moderate to severe obstructive sleep apnea syndrome. Hypertens Res 2007; 30: 669–676.
Ohike Y, Kozaki K, Iijima K, Eto M, Kojima T, Ohga E, Santa T, Imai K, Hashimoto M, Yoshizumi M, Ouchi Y . Amelioration of vascular endothelial dysfunction in obstructive sleep apnea syndrome by nasal continuous positive airway pressure. Possible involvement of nitric OXIDE and asymmetric NG,NG-dimethylarginine. Circ J 2005; 69: 221–226.
Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, Seeger W, Grimminger F . Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med 2000; 162: 566–570.
Yamauchi M, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Suzuki T, Kimura H . Oxidative stress in obstructive sleep apnea. Chest 2005; 127: 1674–1679.
Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK . Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens 1999; 17: 61–66.
Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, Somers VK . Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 2002; 105: 2462–2464.
Kokturk O, Ciftci TU, Mollarecep E, Ciftci B . Elevated C-reactive protein levels and increased cardiovascular risk in patients with obstructive sleep apnea syndrome. Int Heart J 2005; 46: 801–809.
Ishikawa J, Hoshide S, Eguchi K, Ishikawa S, Pickering TG, Shimada K, Kario K . Increased low-grade inflammation and plasminogen-activator inhibitor-1 level in nondippers with sleep apnea syndrome. J Hypertens 2008; 26: 1181–1187.
Ohga E, Nagase T, Tomita T, Teramoto S, Matsuse T, Katayama H, Ouchi Y . Increased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndrome. J Appl Physiol 1999; 87: 10–14.
Wilcox I, Grunstein RR, Hedner JA, Doyle J, Collins FL, Fletcher PJ, Kelly DT, Sullivan CE . Effect of nasal continuous positive airway pressure during sleep on 24-h blood pressure in obstructive sleep apnea. Sleep 1993; 16: 539–544.
Faccenda JF, Mackay TW, Boon NA, Douglas NJ . Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea–hypopnea syndrome. Am J Respir Crit Care Med 2001; 163: 344–348.
Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Sullivan CE, Peter JH . Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107: 68–73.
Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling JR, Davies RJ . Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002; 359: 204–210.
Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK . Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation 1999; 100: 2332–2335.
Martin JM, Carrizo SJ, Vicente E, Agusti AGN . Long-term cardiovascular outcomes in men with obstructive sleep apnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365: 1046–1053.
Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med 1997; 157: 2413–2446.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL, Jones DW, Materson BJ, Oparil S, Wright Jr JT, Roccella EJ, National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JNC7—Complete Version. Hypertension 2003; 42: 1206–1252.
Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B, Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25: 1105–1187.
Acknowledgements
The authors are supported by European Union LSHM-CT-2006-037093 Ingenious grant, and by Foundation for Polish Science TEAM/2008-2/5 and MISTRZ 8/2008 grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wolf, J., Hering, D. & Narkiewicz, K. Non-dipping pattern of hypertension and obstructive sleep apnea syndrome. Hypertens Res 33, 867–871 (2010). https://doi.org/10.1038/hr.2010.153
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2010.153
Keywords
This article is cited by
-
Obstructive sleep apnea and non-dipper: epiphenomena or risks of Alzheimer’s disease?: a review from the HOPE Asia Network
Hypertension Research (2024)
-
Non-Dipping Blood Pressure or Nocturnal Hypertension: Does One Matter More?
Current Hypertension Reports (2024)
-
Statement on chronotherapy for the treatment of hypertension: consensus document from the Korean society of hypertension
Clinical Hypertension (2023)
-
Sleep Apnea Syndrome (SAS) Clinical Practice Guidelines 2020
Sleep and Biological Rhythms (2022)
-
Does treatment-resistant hypertension exist in children? A review of the evidence
Pediatric Nephrology (2020)