Abstract
Hypertension is a disease which affects over 26.4% of the world adult population, therefore novel approaches to the prevention and treatment of this disease need to be examined. Previous studies from our and other laboratories have shown that treatment of spontaneously hypertensive rats (SHR) and Dahl salt-sensitive rats with a renin–angiotensin system (RAS) inhibitor during the ‘critical period’ in hypertension development results in prevention of the later development of hypertension. In humans, Julius et al. reported similar findings in the landmark TROPHY study. Recently, we reported that ‘pulse’ treatment of SHR with high-dose angiotensin receptor blocker (ARB) is effective in causing sustained reduction of already established hypertension, even when the treatment was started after the ‘critical period’. These results suggest the possibility that ‘regression’ of established hypertension may become feasible, and we have started a prospective, multicenter clinical study (STAR CAST study) to examine this possibility. In our animal studies, we found that treatment of rats during the ‘critical period’ with an ARB inhibits the development of renal arteriolar hypertrophy. Moreover, a high-dose angiotensin blocker caused a remarkable reversal of renal arteriolar hypertrophy in SHR, which was associated with changes in microvascular MMP expression. These results suggest that changes in the renal microvasculature may have an important role in the mechanisms of hypertension prevention and regression by ARB.
Similar content being viewed by others
Introduction
Hypertension is a disease which affected 26.4% of the world adult population in 2000, and the prevalence is projected to increase to 29.2% by the year 2025.1 Hypertension is a major risk factor for cardiovascular disorders such as stroke, heart failure, vascular disease and end-stage renal disease, and is a leading cause of morbidity and mortality.
During the last 50 years, the introduction of new drugs with fewer side effects has greatly improved the treatment of hypertension. In particular, the introduction of calcium channel blockers (CCB), followed by inhibitors of the renin–angiotensin system (RAS) have resulted in a greater efficacy/side effect profile, so that these agents are now the most commonly prescribed medications in Japan.2
Unfortunately, these advances have not always resulted in reduction of cardiovascular disease throughout the world. For example, the decline in cardiovascular mortality appears to be slowing down in Japan.3 Moreover, the incidence of end-stage renal disease attributed to hypertensive nephrosclerosis is still increasing.4 One of the obstacles may be the major gap between the prevalence of hypertension, awareness of hypertension and compliance with hypertension treatment.5
These epidemiological data suggest that new and innovative approaches to hypertension therapy need to be considered, to optimize hypertension therapy. The International Society of Hypertension recently published the Fukuoka statement (global challenge for overcoming high blood pressure),6 in which the importance of prevention of hypertension was emphasized. In this review, we summarize the main results of animal experiments to examine the mechanisms of hypertension prevention by RAS inhibitors, and their correlation with recent clinical studies on hypertension prevention. We also examine experimental evidence that ‘regression’ of established hypertension may be feasible, which could result in the ultimate research goal: a ‘cure’ for hypertension.
Definitions of ‘prevention’ and ‘regression’ of hypertension
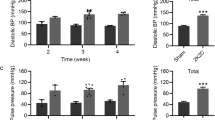
One conceptual definition of hypertension is the level of blood pressure, above which there is a major increase in cardiovascular risk. In international guidelines of hypertension, the numerical threshold of hypertension is usually defined as a systolic blood pressure of 140 mm Hg and/or a diastolic blood pressure of 90 mm Hg. Depending on the blood pressure, hypertension has been further subdivided into different grades as shown in Figure 1. In the 2009 Japanese Society of Hypertension guidelines, blood pressures which are above normal, but below Grade I hypertension are referred to as high-normal blood pressure in the guidelines of the Japanese Society of Hypertension,7 whereas both normal and high-normal blood pressure hypertension correspond to prehypertension in the Joint National Committee JNC-7 guidelines.8
The natural history of hypertension has been well characterized, and it is known that the incidence of hypertension increases greatly during middle age in both men and women. Moreover, hypertension is progressive: the systolic blood pressure of an individual patient rises progressively over time, so that median values of systolic blood pressure in the population increases at every age. Indeed, in the Trial of Preventing Hypertension (TROPHY) study it was reported that 63% of patients with prehypertension would become hypertensive within 4 years.9 Although this figure is greater than the Framingham study,10 it is clear that all patients must pass from normal blood pressure to high-normal blood pressure before progressing to fully established hypertension. Therefore in this review, hypertension ‘prevention’ will be defined as the inhibition of progression of the blood pressure from high-normal blood pressure to Grade I hypertension. Conversely, hypertension ‘regression’ will be defined as the reversal of blood pressure levels from Grade I hypertension back to high-normal levels.
Animal models of hypertension prevention
It has been shown by several groups, including our own, that treatment of young spontaneously hypertensive rats (SHR) with a RAS inhibitor can suppress the development of hypertension. Studies by the group of Harrap et al. demonstrated that treatment of SHR from age 6–10 weeks with an angiotensin-converting enzyme (ACE) inhibitor resulted in the sustained suppression of hypertension at age 25 weeks, whereas treatment from age 6–7 weeks did not.11, 12 Studies from the group of Berecek suggested that these results could result from a decrease in AVP levels.13, 14 Similar studies have been performed by other laboratories using both ACE inhibitors,15, 16 and angiotensin receptor blockers (ARBs).17, 18
In our initial studies, we found that treatment of stroke-prone SHR with an ACE inhibitor from age 3–10 weeks resulted in a sustained suppression of blood pressure, whereas such an effect was not found with the vasodilator hydralazine.19 Exactly the same results were found with an ARB, suggesting that this effect could be explained by the inhibitory actions of ACE inhibitors and ARB on the RAS. We also found that the development of renal injury was also suppressed in this model.
To examine if the effects of RAS inhibitors to suppress the development of hypertension was specific to the SHR and its related strains, we next performed studies on the Dahl salt-sensitive rat, which is a model of salt-sensitive hypertension with a low renin profile.20 We found that treatment of Dahl salt-sensitive rats with an ARB during the same ‘critical period’ (age 3–10 weeks) prevented the later development of salt-induced hypertension in this model even when the ARB treatment had been discontinued, and also a partial attenuation of renal injury induced by salt loading. These studies showed that the prolonged effects of transient treatment of RAS inhibitors are not specific to the SHR model, and can be seen in other strains.
The ‘RAS block memory’ phenomenon and ‘reno-vascular amplifier’ hypothesis
To examine the mechanisms of these long-lasting effects of RAS blockade (which we later called the ‘RAS block memory’ phenomenon), we performed further studies on the SHR/L-NAME model, which is a model of accelerated hypertension characterized by marked renal injury.21 In this model, the rats were treated with a RAS inhibitor (ACE inhibitor or ARB), or a vasodilator (hydralazine), or a CCB (nitrendipine) during the ‘critical period’ from age 3–10 weeks. Medications were discontinued at age 10 weeks, and the rats observed without treatment for two months. At age 18 weeks, the rats were administered the NO synthase inhibitor L-NAME in the drinking water for 3 weeks to induce renal injury, and killed at age 21 weeks. Interestingly, the rats treated with a RAS inhibitor had reduced vascular injury (arterial hypertrophy, endothelial thickening and lumen narrowing) compared with vasodilator- or CCB-treated rats, and reduced renin mRNA, probably due to attenuation of the intrarenal vascular injury and renal ischemia induced by L-NAME. To explain all these experimental findings, we proposed a ‘reno-vascular amplifier’ mechanism for the development of hypertension and renal injury in this model. High blood pressure is known to cause vascular hypertrophy in the resistance vessels, which predominantly consists of inward ‘eutrophic’ remodeling. When this remodeling is accentuated, as in the SHR/L-NAME model, glomerular perfusion decreases, which results in increased synthesis of renin and activation of the RAS. These changes cause a further increase in the blood pressure, resulting in a vicious cycle, which causes accelerated hypertension. RAS inhibitors can block this vicious cycle by attenuating both the increase in blood pressure, and importantly, by decreasing the vascular hypertrophy of the resistance arteries. This could explain why the effects of ARB were prolonged, resulting in the ‘RAS block memory’ phenomenon.21 This hypothesis was supported by experiments in which we administered the agonist angiotensin II during the ‘critical period’ from age 4–8 weeks, after which all treatments were discontinued. Rats, which had been transiently exposed to angiotensin II during the ‘critical period’ were found to have elevated values of blood pressure at the later period which were 10–20 mm Hg higher than rats which had been exposed to saline vehicle. Moreover, these rats were more susceptible to the subsequent development of renal vascular injury, and increased renin synthesis at a later time point (age 18 weeks) and to have a much higher mortality after L-NAME administration.21 In other words, the effects of angiotensin II administration were the opposite of the effects of ARB, and were found to cause an acceleration of the ‘reno-vascular amplifier’ in this model of accelerated hypertension and renal injury.
Clinical studies of hypertension prevention
The results of animal studies on hypertension prevention have been supported clinically by the TROPHY study.9 In this prospective, randomized, multi-center study designed by Julius et al.,9 patients with prehypertension and systolic blood pressure of 130–139 mm Hg and/or diastolic blood pressure of 85–89 mm Hg were randomized to placebo or the ARB candesartan cilexetil (16 mg/day) for 2 years, then both groups were switched to placebo for the next 2 years. The primary end point was the development of hypertension. As in the animal studies, the treatment with ARB caused a suppression of the development of hypertension, not only during the active treatment period (first 2 years), but even after the active treatment had been discontinued. The absolute risk reduction at the end of 2 or 4 years was 26.8 and 9.8%, respectively, whereas the corresponding values of relative risk reduction were 66.3 and 15.6%. Changes in the systolic blood pressure at the end of the study were small (2 mm Hg), but statistically significant.
In a smaller trial from the Denmark (the DHyPP) study, the investigators were not able to find a statistically significant effect of treatment with an ARB for 1 year on subsequent values of blood pressure measured by ABPM.22 However, the number of subjects enrolled was much smaller than the TROPHY study, so it is unclear whether a statistically significant change could have been detected with a greater number of patients. Moreover, the subjects had diastolic blood pressures of 85 mm Hg or less, and so were not prehypertensive.
In the Prevention of Hypertension in Patients with High-Normal Blood Pressure with the ACE Inhibitor Ramipril study, the investigators examined whether 3-year treatment of patients with high-normal blood pressure with the ACE inhibitor ramipril would prevent or delay the progression to hypertension.23 Hypertension was found to develop in 30.7% of the ramipril-treated patients compared with 42.9% of the controls, a relative risk reduction of 34.4%. These results suggested that treatment of prehypertensive patients with ACE inhibitor could prevent the development of hypertension in this population. The investigators did not examine if the decrease in blood pressure persisted after the end of the treatment.
Animal models of hypertension regression
Previous studies suggested that treatment of SHR after the ‘critical period’ would not result in sustained suppression of hypertension. However, studies by the group of Smallegange et al.24 showed that transient treatment of SHR with an ACE inhibitor together with a low-salt diet could cause sustained reduction of blood pressure, even after the treatment was discontinued. Moreover, the same group reported that the reduction of blood pressure could be transferred to a different SHR, if the kidney was transplanted to another rat. The reduction of blood pressure was also found to be associated with decreased renal vascular resistance.
Recently, our group showed that ‘pulse’ treatment with high-dose ARB is effective in causing 30–40 mm Hg regression of established hypertension in SHR.25 Similar results were found with both ARB and ACE inhibitor, whereas no such effect was found with a CCB or vasodilator. Importantly, 4 months after the ‘pulse’ treatment, not only the blood pressure, but also cardiac and aortic hypertrophy were significantly reduced in the rats, which had been ‘pulse’-treated with ARB or ACE inhibitor, probably as a consequence of the regression of hypertension.
Clinical studies of hypertension regression
To our knowledge, there have been no clinical studies, which were designed to address the question whether regression of hypertension (that is, reversal of Grade 1 hypertension to high-normal blood pressure) is feasible in humans. For this reason, we have recently designed and started a preliminary prospective, multi-center study (STAR CAST) to examine the effects of a 1 year treatment with an ARB or CCB on regression of hypertension.26 If the results are encouraging, we hope to perform further studies using high or ultrahigh doses of ARB in patients with hypertension or chronic kidney disease.
Role of the renal microvascular remodeling in the pathogenesis of hypertension
Hypertension is associated with increased peripheral arterial resistance, and most of the resistance is developed in the resistance arteries of the microvasculature, which includes both arterioles and small arteries with diameters <400 μm. The importance of the microvasculature in the pathogenesis and maintenance of hypertension was originally proposed by Folkow,27 who pointed out that a vicious cycle exists between increased blood pressure and vascular hypertrophy. According to this hypothesis, hypertension may be initiated by a specific fast-acting pressor mechanism (for example, angiotensin II) that increases blood pressure and initiates a positive feedback loop that induces vascular hypertrophy and maintains the hypertension. The hypothesis was later refined by Lever and Harrap,28 who proposed further elements: an abnormal or ‘reinforced’ hypertrophic response to pressure, and an increase of a humoral agent that causes hypertrophy directly.
Animal studies have provided evidence to support these hypotheses (for extended reviews, see Skov and Mulvany,29 Intengan and Schiffrin,30 Feihl et al.31). Hemodynamic studies indicated that increased renal vascular resistance is already present in the prehypertensive state in SHR.32, 33 The increase in resistance appears to be more marked in the renal vasculature compared with the increase seen in other vascular beds.29 As renal resistance resides predominantly within the afferent arterioles,34 the increased renal resistance could be caused by narrowing of the afferent arteriolar lumen. Indeed, morphometric studies on the afferent arteriole of SHR and WKY have confirmed that afferent arteriolar diameters are smaller in SHR compared with WKY, whereas efferent arteriole diameters are comparable between the two strains.35, 36 Importantly, these differences are already seen in the 4-week-old SHR, even before blood pressure is significantly increased compared with WKY controls.35 Moreover, Simon et al.37, 38 showed that a 2% NaCl high-salt diet in combination with Ang II infusion increased wall-lumen ratios of small resistance arteries, preglomerular structural vascular resistance and blood pressure in Sprague–Dawley rats. These results are compatible with the notion that restriction of sodium intake has an important role in efforts to prevent and control hypertension.7
Cross-breeding studies have provided further evidence of a role for a renal ‘structural factor’ in the pathogenesis of hypertension in SHR. It is well recognized that transplantation of kidneys from SHR to WKY results in transfer of the hypertension, whereas transplantation from WKY to SHR results in normalization of the blood pressure,39, 40, 41 consistent with a central role for the kidney in the pathogenesis of hypertension. To extend these findings, Norrelund et al.42 performed studies in which the progeny of a cross between hypertensive and normotensive animals were themselves crossed to produce an F2-generation. Interestingly, it was found that a narrowed afferent arteriole in young F2-SHR/WKY was associated with the later development of high blood pressure, suggesting a role for this phenotype in the pathogenesis of hypertension. Recently, the group of Coffman and Crowley43 showed that transplantation of kidneys from AT1a receptor-deficient mice to wild-type mice almost completely abolished hypertension induced by Ang II infusion, whereas transplantation of kidneys from wild-type mice to AT1a receptor-deficient mice restored the blood pressure response. These results provide further strong evidence for the important role of the kidney in regulating systemic blood pressure.
In human patients, hypertension has different effects on the vascular morphology of large arteries and resistance arteries.44 In the large elastic and muscular arteries, elevated blood pressure leads to an increase in the diameter and the intima-media thickness. In the case of the small muscular resistance arteries and arterioles, essential hypertension is associated with a decrease in the lumen diameter and an increase in the media-to-lumen ratio of resistance vessels.29, 30 With mild-to-moderate hypertension, this alteration appears to involve a rearrangement of the vascular structure, such that the arterial wall cross-sectional area is not changed, whereas the lumen is reduced (‘eutrophic remodeling’). In the case of severe hypertension or in forms of secondary hypertension, hypertrophy of the vascular wall results in an increase in the arterial wall cross-sectional area together with a decrease in lumen diameter (‘hypertrophic remodeling’). It has been suggested that small artery remodeling may contribute to the morning surge in blood pressure in humans.45
Composition of the extracellular matrix (ECM) in the vasculature, and remodeling during hypertension
Eutrophic remodeling involves restructuring of the vascular wall, so that smooth muscle cells are aligned more closely and encircle the lumen more tightly without a change in the volume of the media.44 This remodeling requires a complex restructuring of the ECM with increased ECM deposition in the inner lumen of the arteriole, together with degradation of ECM in the periphery.
The ECM is a complex mixture of macromolecules, which may be broadly classified into three major types: structural proteins such as collagen and elastin, specialized and adhesive proteins such as fibronectin and laminin, and proteoglycans/glycosaminoglycans. In the arteries, types I and III collagens form the bulk of vascular collagens (60 and 30%, respectively), whereas the remaining 10% includes types IV, V, VI, VIII, XII, XIV collagens.46 Type I collagen is most prevalent in the adventitia of the rat aorta, whereas type III collagen is found in the media and adventitia.47 In humans, types I and III have been detected in the intima media and adventitia.48 In the large arteries, elastin can comprise up to 50% of its dry weight,49 however, in the resistance arteries, elastin is much less abundant and is localized mainly in the internal and external elastic lamina. The fibrous proteins collagen and elastin are embedded in a gel-like ground substance composed of proteoglycans/glycosaminoglycans.50 Both structural proteins and proteoglycans are known to be associated with adhesive proteins such as fibronectin, vitronectin, laminin and thrombospondin.51 The adhesive proteins interact with other ECM molecules, so that the ECM surrounding the body’s cells form a structural scaffold which maintains the arterial framework.
When hypertension develops, the first ECM response to the elevated wall stress is an increase in elastin synthesis.52 Pressure-induced stretch may trigger this response, because cultured VSMC can be elicited to increase elastin synthesis by applying stretch to culture dishes with deformable bottoms.53 Collagen, the other fibrous component of the vascular ECM, is also increased after the initiation of hypertension.54 In the peripheral resistance arteries of the SHR, vessel wall stiffness is increased and this is associated with an increased volume density of collagen, as well as an increased collagen/elastin ratio. The collagen/elastin ratio has also been shown to be increased in human resistance arteries from mild essential hypertensive patients.55, 56, 57 Moreover, vascular proteoglycan synthesis is also increased by high blood pressure.58 These changes may contribute to the inward remodeling of the arterioles found in hypertension.44
Concerning ECM degradative pathways, expression of MMP-2, TIMP-1 and TIMP-2 has been reported in normal arteries.59 Changes in MMP activity and expression have been reported as a result of hypertension. When porcine arteries ex vivo are subjected to an elevation of transmural pressure, an increase in MMP-2 and MMP-9 activities are seen.60 Similar results have been reported in vitro, namely cyclical mechanical strain to simulate blood pressure changes results in an increase in MMP-2 activity in human VSMC.61 Moreover, in young SHR, MMP-1 activity was reported to be increased in the mesenteric arterial bed is decreased before hypertension was established. Taken together, these data suggest important roles for MMPs in the process of vascular remodeling in hypertension.44
Role of renal microvascular protection in the prevention and regression of hypertension by RAS inhibitors
Recently, we examined the morphological effects of treatment with an ARB or CCB during the ‘critical period’ on renal small artery structure in SHR. SHR were treated with an ARB (candesartan cilexetil) or CCB (nitrendipine) from age 3–10 weeks, and killed at age 10 weeks. In this simple experiment, the arteriolar hypertrophy was found to be significantly reduced in the ARB-treated rats compared with the CCB-treated rats, despite similar reductions in blood pressure (Figure 2). Similar results have been reported by other groups using ACE inhibitors in both animal models,16, 62 and humans.63, 64
We also examined renal arteriolar structure in rats treated with high-dose ‘pulse’ ARB therapy, and found a remarkable reversal of the arteriolar hypertrophy found in SHR treated with ARB, whereas this effect was not seen with CCB (Figure 3). Interestingly, these findings were marked in the small renal arteries (diameter 30–100 μm) compared with small arteries from other vascular beds.25 To examine potential mechanisms of these changes, we compared the gene expression profile of kidneys treated with ARB vs. kidneys treated with CCB. The differences in expression of a total of 28 000 genes in the kidneys of SHR treated with ARB or CCB were examined using the Affymetrix rat 230 2.0 gene expression array (Affymetrix KK, Tokyo, Japan). In all, 1345 genes were elevated in the ARB-treated rats compared with CCB-treated rats, whereas 5671 were reduced. Several ECM-related genes were elevated in the ARB-treated rats, whereas MMP-9, TIMP-2 and TIMP-3 gene expressions were decreased in the ARB-treated group (Table 1). These differences were confirmed by real time RT-PCR. To examine if these changes in MMP expression could be involved in the observed reversal of renal arteriolar hypertrophy by ARB, we examined the activities of different MMPs in the renal microvasculature using a highly sensitive in situ zymography method. We found that MMP-13 activity was markedly increased by ARB but not by CCB.25 As MMP-13 is known to be the predominant type I collagenase in rodents, these results are compatible with an important role for MMPs in the actions of ARB to cause reversal of renal arteriolar hypertrophy, and subsequent remodeling of the renal microvasculature (Figure 4).
Hypothesis for the important role of renal arteriolar remodeling in the prevention and regression of hypertension. (a) Prevention of renal arteriolar remodeling as a potential mechanism for ARB/angiotensin-converting enzyme inhibitor (ACEI)-induced prevention of hypertension (b) Reversal of renal arteriolar remodeling as a potential mechanism for ‘pulse’ ARB/ACEI-induced regression of hypertension. Abbreviations as in Figure 2.
Conclusion
It has been suggested for a long time that hypertrophy of the renal small arteries may have an important role in the pathogenesis of hypertension. Recent data from our and other laboratories suggest that the effects of RAS inhibitors to prevent and reverse small artery hypertrophy may be unique among the antihypertensive agents. These ‘protective’ effects of RAS inhibitors on the renal microvasculature may explain the efficacy of these agents not only in preventing the onset of hypertension, and also in inducing regression of hypertension in animal models. It is hoped that further studies on the mechanisms of hypertension prevention and regression may lead to the ultimate research goal: the development of feasible methods for hypertension prevention and regression in humans.
Conflict of interest
The authors declare no conflict of interest.
References
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J . Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365: 217–223.
Saito I, Kawabe H, Tsujioka M, Hirose H, Shibata H . Trends in pharmacologic management of hypertension in Japan one year after the publication of the JSH 2000 guidelines. First Japanese Society of Hypertension. Hypertens Res 2002; 25: 175–178.
Kubo M, Kiyohara Y, Kato I, Tanizaki Y, Arima H, Tanaka K, Nakamura H, Okubo K, Iida M . Trends in the incidence, mortality, and survival rate of cardiovascular disease in a Japanese community: The Hisayama Study. Stroke 2003; 34: 2349–2354.
Nakai S, Masakane I, Akiba T, Iseki K, Watanabe Y, Itami N, Kimata N, Shigematsu T, Shinoda T, Syoji T, Suzuki K, Tsuchida K, Nakamoto H, Hamano T, Marubayashi S, Morita O, Morozumi K, Yamagata K, Yamashita A, Wakai K, Wada A, Tsubakihara Y . Overview of regular dialysis treatment in Japan (as of 31 December 2005). Ther Apher Dial 2007; 11: 411–441.
Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ . Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension 2008; 52: 818–827.
ISH. Global challenge for overcoming high blood pressure: Fukuoka statement. J Hypertens 2006; 29: 934.
JSH. Japanese Society of Hypertension Guidelines for the Management of Hypertension. J Hypertens 2006; 29 (Supplement): S1–S105.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL, Jones DW, Materson BJ, Oparil S, Wright Jr JT, Roccella EJ . The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572.
Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, Kaciroti N, Black HR, Grimm Jr RH, Messerli FH, Oparil S, Schork MA . Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med 2006; 354: 1685–1697.
Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D . Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet 2001; 358: 1682–1686.
Harrap SB, Nicolaci JA, Doyle AE . Persistent effects on blood pressure and renal haemodynamics following chronic angiotensin converting enzyme inhibition with perindopril. Clin Exp Pharmacol Physiol 1986; 13: 753–765.
Harrap SB, Van der Merwe WM, Griffin SA, Macpherson F, Lever AF . Brief angiotensin converting enzyme inhibitor treatment in young spontaneously hypertensive rats reduces blood pressure long-term. Hypertension 1990; 16: 603–614.
Lee RM, Berecek KH, Tsoporis J, McKenzie R, Triggle CR . Prevention of hypertension and vascular changes by captopril treatment. Hypertension 1991; 17: 141–150.
Zhang L, Edwards DG, Berecek KH . Effects of early captopril treatment and its removal on plasma angiotensin converting enzyme (ACE) activity and arginine vasopressin in hypertensive rats (SHR) and normotensive rats (WKY). Clin Exp Hypertens 1996; 18: 201–226.
Giudicelli JF, Freslon JL, Glasson S, Richer C . Captopril and hypertension development in the SHR. Clin Exp Hypertens 1980; 2: 1083–1096.
Christensen KL, Jespersen LT, Mulvany MJ . Development of blood pressure in spontaneously hypertensive rats after withdrawal of long-term treatment related to vascular structure. J Hypertens 1989; 7: 83–90.
Morton JJ, Beattie EC, MacPherson F . Angiotensin II receptor antagonist losartan has persistent effects on blood pressure in the young spontaneously hypertensive rat: lack of relation to vascular structure. J Vasc Res 1992; 29: 264–269.
Gillies LK, Lu M, Wang H, Lee RM . AT1 receptor antagonist treatment caused persistent arterial functional changes in young spontaneously hypertensive rats. Hypertension 1997; 30: 1471–1478.
Nakaya H, Sasamura H, Hayashi M, Saruta T . Temporary treatment of prepubescent rats with angiotensin inhibitors suppresses the development of hypertensive nephrosclerosis. J Am Soc Nephrol 2001; 12: 659–666.
Nakaya H, Sasamura H, Mifune M, Shimizu-Hirota R, Kuroda M, Hayashi M, Saruta T . Prepubertal treatment with angiotensin receptor blocker causes partial attenuation of hypertension and renal damage in adult Dahl salt-sensitive rats. Nephron 2002; 91: 710–718.
Ishiguro K, Sasamura H, Sakamaki Y, Itoh H, Saruta T . Developmental activity of the renin-angiotensin system during the ‘critical period’ modulates later L-NAME-induced hypertension and renal injury. Hypertens Res 2007; 30: 63–75.
Skov K, Eiskjaer H, Hansen HE, Madsen JK, Kvist S, Mulvany MJ . Treatment of young subjects at high familial risk of future hypertension with an angiotensin-receptor blocker. Hypertension 2007; 50: 89–95.
Luders S, Schrader J, Berger J, Unger T, Zidek W, Bohm M, Middeke M, Motz W, Lubcke C, Gansz A, Brokamp L, Schmieder RE, Trenkwalder P, Haller H, Dominiak P . The PHARAO study: prevention of hypertension with the angiotensin-converting enzyme inhibitor ramipril in patients with high-normal blood pressure: a prospective, randomized, controlled prevention trial of the German Hypertension League. J Hypertens 2008; 26: 1487–1496.
Smallegange C, Hale TM, Bushfield TL, Adams MA . Persistent lowering of pressure by transplanting kidneys from adult spontaneously hypertensive rats treated with brief antihypertensive therapy. Hypertension 2004; 44: 89–94.
Ishiguro K, Hayashi K, Sasamura H, Sakamaki Y, Itoh H . ‘Pulse’ treatment with high-dose angiotensin blocker reverses renal arteriolar hypertrophy and regresses hypertension. Hypertension 2009; 53: 83–89.
Sasamura H, Nakaya H, Julius S, Takebayashi T, Sato Y, Uno H, Takeuchi M, Ishiguro K, Murakami M, Ryuzaki M, Itoh H . Short treatment with the angiotensin receptor blocker candesartan surveyed by telemedicine (STAR CAST) study: rationale and study design. Hypertens Res 2008; 31: 1851–1857.
Folkow B . ‘Structural factor’ in primary and secondary hypertension. Hypertension 1990; 16: 89–101.
Lever AF, Harrap SB . Essential hypertension: a disorder of growth with origins in childhood? J Hypertens 1992; 10: 101–120.
Skov K, Mulvany MJ . Structure of renal afferent arterioles in the pathogenesis of hypertension. Acta Physiol Scand 2004; 181: 397–405.
Intengan HD, Schiffrin EL . Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 2001; 38: 581–587.
Feihl F, Liaudet L, Waeber B, Levy BI . Hypertension: a disease of the microcirculation? Hypertension 2006; 48: 1012–1017.
Dilley JR, Stier Jr CT, Arendshorst WJ . Abnormalities in glomerular function in rats developing spontaneous hypertension. Am J Physiol 1984; 246: F12–F20.
Harrap SB, Doyle AE . Genetic co-segregation of renal haemodynamics and blood pressure in the spontaneously hypertensive rat. Clin Sci (Lond) 1988; 74: 63–69.
Arendshorst WJ, Beierwaltes WH . Renal and nephron hemodynamics in spontaneously hypertensive rats. Am J Physiol 1979; 236: F246–F251.
Kimura K, Nanba S, Tojo A, Hirata Y, Matsuoka H, Sugimoto T . Variations in arterioles in spontaneously hypertensive rats. Morphometric analysis of afferent and efferent arterioles. Virchows Arch A Pathol Anat Histopathol 1989; 415: 565–569.
Gattone II VH, Evan AP, Willis LR, Luft FC . Renal afferent arteriole in the spontaneously hypertensive rat. Hypertension 1983; 5: 8–16.
Simon G, Illyes G, Csiky B . Structural vascular changes in hypertension: role of angiotensin II, dietary sodium supplementation, blood pressure, and time. Hypertension 1998; 32: 654–660.
Simon G, Jackel M, Illyes G . Role of angiotensin II, sympathetic stimulation and salt in the development of structural vascular changes in rat kidney. Clin Exp Pharmacol Physiol 2003; 30: 476–481.
Bianchi G, Fox U, Di Francesco GF, Giovanetti AM, Pagetti D . Blood pressure changes produced by kidney cross-transplantation between spontaneously hypertensive rats and normotensive rats. Clin Sci Mol Med 1974; 47: 435–448.
Rettig R, Stauss H, Folberth C, Ganten D, Waldherr B, Unger T . Hypertension transmitted by kidneys from stroke-prone spontaneously hypertensive rats. Am J Physiol 1989; 257: F197–F203.
Patschan O, Kuttler B, Heemann U, Uber A, Rettig R . Kidneys from normotensive donors lower blood pressure in young transplanted spontaneously hypertensive rats. Am J Physiol 1997; 273: R175–R180.
Norrelund H, Christensen KL, Samani NJ, Kimber P, Mulvany MJ, Korsgaard N . Early narrowed afferent arteriole is a contributor to the development of hypertension. Hypertension 1994; 24: 301–308.
Coffman TM, Crowley SD . Kidney in hypertension: Guyton redux. Hypertension 2008; 51: 811–816.
Intengan HD, Schiffrin EL . Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension 2000; 36: 312–318.
Kario K . Preceding linkage between a morning surge in blood pressure and small artery remodeling: an indicator of prehypertension? J Hypertens 2007; 25: 1573–1575.
Jacob MP . Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother 2003; 57: 195–202.
Farquharson C, Robins SP . Immunolocalization of collagen types I and III in the arterial wall of the rat. Histochem J 1989; 21: 172–178.
Shekhonin BV, Domogatsky SP, Muzykantov VR, Idelson GL, Rukosuev VS . Distribution of type I, III, IV and V collagen in normal and atherosclerotic human arterial wall: immunomorphological characteristics. Coll Relat Res 1985; 5: 355–368.
Brooke BS, Bayes-Genis A, Li DY . New insights into elastin and vascular disease. Trends Cardiovasc Med 2003; 13: 176–181.
Kresse H, Schonherr E . Proteoglycans of the extracellular matrix and growth control. J Cell Physiol 2001; 189: 266–274.
Yamada KM . Adhesive recognition sequences. J Biol Chem 1991; 266: 12809–12812.
Keeley FW, Bartoszewicz LA . Elastin in systemic and pulmonary hypertension. Ciba Found Symp 1995; 192: 259–273; discussion 273–8.
Sutcliffe MC, Davidson JM . Effect of static stretching on elastin production by porcine aortic smooth muscle cells. Matrix 1990; 10: 148–153.
Xu C, Zarins CK, Bassiouny HS, Briggs WH, Reardon C, Glagov S . Differential transmural distribution of gene expression for collagen types I and III proximal to aortic coarctation in the rabbit. J Vasc Res 2000; 37: 170–182.
Intengan HD, Thibault G, Li JS, Schiffrin EL . Resistance artery mechanics, structure, and extracellular components in spontaneously hypertensive rats : effects of angiotensin receptor antagonism and converting enzyme inhibition. Circulation 1999; 100: 2267–2275.
Sharifi AM, Li JS, Endemann D, Schiffrin EL . Effects of enalapril and amlodipine on small-artery structure and composition, and on endothelial dysfunction in spontaneously hypertensive rats. J Hypertens 1998; 16: 457–466.
Intengan HD, Deng LY, Li JS, Schiffrin EL . Mechanics and composition of human subcutaneous resistance arteries in essential hypertension. Hypertension 1999; 33: 569–574.
Lipke DW, Couchman JR . Increased proteoglycan synthesis by the cardiovascular system of coarctation hypertensive rats. J Cell Physiol 1991; 147: 479–486.
Galis ZS, Khatri JJ . Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 2002; 90: 251–262.
Chesler NC, Ku DN, Galis ZS . Transmural pressure induces matrix-degrading activity in porcine arteries ex vivo. Am J Physiol 1999; 277: H2002–H2009.
O’Callaghan CJ, Williams B . Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: role of TGF-beta (1). Hypertension 2000; 36: 319–324.
Freslon JL, Giudicelli JF . Compared myocardial and vascular effects of captopril and dihydralazine during hypertension development in spontaneously hypertensive rats. Br J Pharmacol 1983; 80: 533–543.
Schiffrin EL, Deng LY, Larochelle P . Effects of antihypertensive treatment on vascular remodeling in essential hypertensive patients. J Cardiovasc Pharmacol 1994; 24 (Suppl 3): S51–S56.
Thybo NK, Stephens N, Cooper A, Aalkjaer C, Heagerty AM, Mulvany MJ . Effect of antihypertensive treatment on small arteries of patients with previously untreated essential hypertension. Hypertension 1995; 25: 474–481.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sasamura, H., Hayashi, K., Ishiguro, K. et al. Prevention and regression of hypertension: role of renal microvascular protection. Hypertens Res 32, 658–664 (2009). https://doi.org/10.1038/hr.2009.85
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2009.85
Keywords
This article is cited by
-
Clinical significance of ‘cardiometabolic memory’: a systematic review of randomized controlled trials
Hypertension Research (2017)
-
Pre-hypertension: another 'pseudodisease’?
BMC Medicine (2013)
-
Vaccination against the angiotensin type 1 receptor for the prevention of L-NAME-induced nephropathy
Hypertension Research (2012)
-
‘Memory’ and ‘legacy’ in hypertension and lifestyle-related diseases
Hypertension Research (2012)