Abstract
Pharmacological evidence suggests that angiotensin II type 1 (AT1) receptors are involved in the regulation of cardiovascular response to emotional stress and reinforcing effect of dietary salt on this response. In this study, we examined the effect of genetic deletion of AT1A receptors on the cardiovascular effects of stress and salt in mice. AT1A receptor knockout (AT1A−/−) and wild-type (AT1A+/+) mice were implanted with telemetry devices and placed on a normal (0.4%) or high (3.1%) salt diet (HSD). Resting blood pressure (BP) in AT1A−/− mice (84±3 mm Hg) was lower than in AT1A+/+ mice (107±2 mm Hg). Negative emotional (restraint) stress increased BP by 33±3 mm Hg in AT1A+/+ mice. This response was attenuated by 40% in AT1A−/− mice (18±3 mm Hg). Conversely, the BP increase caused by food presentation and feeding was similar in AT1A−/− (25±3 mm Hg) and AT1A+/+ mice (26±3 mm Hg). HSD increased resting BP by 14±4 mm Hg in AT1A−/− mice without affecting it significantly in AT1A+/+ mice. Under these conditions, the pressor response to restraint stress in AT1A−/− mice (30±3 mm Hg) was no longer different from that in wild-type animals (28±3 mm Hg). The BP response to feeding was not altered by HSD in either AT1A−/− or AT1A+/+ mice (25±2 and 27±3 mm Hg, respectively). These results indicate that AT1A receptor deficiency leads to a reduction in BP reactivity to negative emotional stress, but not feeding. HSD can selectively reinforce the cardiovascular response to negative stress in AT1A−/− mice. However, there is little interaction between AT1A receptors, excess dietary sodium and feeding-induced cardiovascular arousal.
Similar content being viewed by others
Introduction
Increased cardiovascular reactivity to psycho-emotional stress is considered to be a risk factor for hypertension and heart disease.1, 2 The regulation of cardiovascular reactivity is a complex phenomenon involving several transmitters and pathways at different levels of the central and peripheral nervous system. Recent pharmacological studies, however, indicate that the activation of angiotensin II type 1 (AT1) receptors, and specifically those in the dorsomedial hypothalamus (DMH) and rostral ventrolateral medulla (RVLM), is required for full expression of sympathetic cardiovascular responses to various psychoemotional stressors in rats and rabbits.3, 4, 5, 6, 7 Nonetheless, the effect of genetic deficiency of AT1 receptors on cardiovascular reactivity to stress is yet to be determined.
Apart from aversive events, appetitive stimuli are capable of inducing a distinct, sympathetically mediated rise in blood pressure and heart rate in animals.8, 9 Likewise, activated positive emotions, and in particular those initiated by personally relevant stimuli, are associated with increased blood pressure and heart rate in humans. In contrast to aversive events, these positive emotions are often linked to health protective responses.10, 11 Intriguingly, our initial studies showed that AT1 receptors seem to be less essential in modulating cardiovascular arousal associated with appetitive events,12 indicating that these receptors could be a selective therapeutic target for stress-related cardiovascular disorders.
It is well established that high dietary salt can facilitate the cardiovascular response to emotional stress in genetically predisposed animals and humans.13, 14, 15 Central AT1 receptors have been implicated in this reinforcing effect of dietary salt on the stress response in salt-sensitive rat models of hypertension.16, 17 Conversely, the effect of dietary salt loading on cardiovascular arousal associated with appetitive behavior remains to be determined, as is the role of AT1 receptors in this effect.
In this study, we examined the cardiovascular response to aversive and appetitive stimuli in genetically engineered mice lacking one of the two main isoforms of murine AT1 receptor (AT1A). This receptor, which is pharmacologically indistinguishable from AT1B receptor, has been shown to play a crucial role in the cardiovascular effects of angiotensin II.18, 19 We also determined the effect of dietary salt loading on cardiovascular arousal caused by aversive and appetitive stimuli in these animals. We used a telemetry blood-pressure monitoring system to take into account the confounding influence of locomotion on blood pressure,20, 21 which might mask or alter the effect of emotional arousal on cardiovascular function.
Methods
General preparations
The experiments were carried out using 2–3-month-old male AT1A receptor knockout (AT1A−/−) and wild-type (AT1A+/+) mice in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. AT1A−/− mice and their control strain were bred at the animal facilities of the Baker Heart Research Institute. Mice were originally obtained from Prof. T Walther (animal facilities of Charité, Berlin, Germany). F2 generation mice were produced from crosses of (129 × C57BL/6J) F1 AT1A−/+ parents. Mice were then maintained on a C57BL/6 background and genotyped using PCR analysis of DNA isolated from tail biopsies.
Under fluothane anesthesia, mice were implanted with TA11PA-C10 telemetry probes to measure blood pressure and locomotor activity (Data Sciences International, St Paul, MN, USA) as described elsewhere.22 Each mouse was then housed individually. After a 1-week recovery period, mice were placed on a normal (0.4%) or high (3.1%) salt diet using the crossover design. Stress tests were conducted between 1100 and 1600 hours. Mice were maintained on a 12:12-h light–dark cycle (lights off at 1800 hours) with water and food ad libitum.
Measurement of BP, heart rate and locomotor activity
During the experiment, pulsatile arterial pressure and gross locomotor activity were monitored continuously, and were sampled at 1000 Hz using an analog-to-digital data acquisition card, and the beat-to-beat mean arterial pressure (MAP) and heart rate (HR) were detected online and analyzed using a program written in Labview (National Instruments, Austin, TX, USA), as described earlier.21
Emotional stress and feeding tests
The restraint and feeding tests were conducted as described earlier.21 Briefly, the restraint test consisted of placing a mouse in a well-ventilated plexiglass restrainer for 5 min with a sliding back plate to confine the mouse without applying physical pressure on it. Feeding was initiated by placing a piece of almond (∼0.5 g) in the mouse home cage. The tests were randomized and separated by at least 1-h recovery periods to ensure full recovery of cardiovascular parameters.
Cardiovascular reactivity to locomotor activity
To calculate the reactivity index, average MAP, HR and locomotor activity values were calculated over 30-s intervals, and the activity score was logarithmically transformed to correct for positive skew.20, 21, 23 For each mouse, least-squares regression slopes for the relationships between MAP and activity, and HR and activity were calculated over 10-min periods (which included 5-min control and stress periods of 10 data points for each period) using GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Statistical analysis
All values are expressed as mean±s.e.m. Data were analyzed by two-way analysis of variance (ANOVA) to determine the effects of AT1A receptor deficiency and stress on cardiovascular parameters and locomotion. The relationships between cardiovascular reactivity and physical activity (regression slopes) were compared by analysis of covariance (ANCOVA) using GraphPad Prism. Statistical significance was set at a value of P<0.05.
Results
Restraint stress
Resting pre-stress levels of MAP in AT1A−/− mice were lower by 25±5 mm Hg than in AT1A+/+ mice (P<0.001), whereas basal HR and locomotor activity did not differ between groups (Table 1). Restraint stress caused prompt pressor (33±3 mm Hg) and tachycardic (203±19 b.p.m.) responses in AT1A+/+ mice (Figure 1). There was also a moderate increase in locomotor activity (245±78 AU), as animals typically tried to escape from the restrainer. The pressor response to restraint stress was attenuated by 40% in AT1A−/− mice (20±3 mm Hg, P=0.01). The tachycardic and locomotor responses to restraint stress were similar between AT1A+/+ and AT1A−/− mice (Figure 1).
(a) Cardiovascular and locomotor response to restraint stress in AT1A−/− and AT1A+/+ mice. Each dot represents an average over a 30-s period. In the right panels, the mean values of mean arterial pressure (MAP), heart rate (HR) and activity over the last 4 min of stress exposure and 5 min immediately before stress in each animal were used to calculate the average responses. *P=0.01 vs. AT1A+/+ mice; AU, arbitrary units. (b) Relationship between MAP and locomotion during the restraint test in AT1A−/− and AT1A+/+ mice.
There was a correlation between MAP and locomotion in both AT1A−/− mice (r=0.35, P<0.001) and AT1A+/+ mice (r=0.51, P<0.001; Figure 1). Likewise, HR and locomotion were significantly correlated in both groups during the test (data not shown). MAP responsiveness to locomotion was not statistically different between AT1A−/− mice (5.9±1.0 mm Hg per log10AU) and AT1A+/+ mice (8.2±1.0 mm Hg per log10AU). Similarly, HR responsiveness to locomotion did not differ between groups (data not shown).
Food presentation and feeding
Pre-feeding levels of MAP in AT1A−/− mice were lower by 24±4 mm Hg than in AT1A+/+ mice (P<0.001), whereas basal HR and locomotor activity did not differ between groups (Table 1). There was no difference between corresponding basal parameters before feeding and restraint tests (Table 1).
The presentation and eating of palatable food (almond) in AT1A+/+ mice elicited prompt behavioral arousal accompanied by pressor and tachycardic responses (26±3 mm Hg and 113±26 b.p.m., respectively; Figure 2). These responses were not altered in AT1A−/− mice (25±3 mm Hg and 99±9 b.p.m., respectively), neither were locomotor responses (Figure 2).
(a) Cardiovascular and locomotor response to feeding in AT1A−/− and AT1A+/+ mice. Abbreviations as in Figure 1. (b) Relationship between MAP and locomotion during feeding in AT1A−/− and AT1A+/+ mice.
There was a significant correlation between MAP and locomotion in both AT1A−/− mice (r=0.52, P<0.01) and AT1A+/+ mice (r=0.39, P<0.01) during the test (Figure 2). The responsiveness of MAP to locomotor activity was similar between AT1A−/− and AT1A+/+ mice (7.0±0.8 and 5.6±1.0 mm Hg per log10AU, respectively), as was HR responsiveness to locomotion (data not shown).
Effect of high salt intake on restraint stress response
High salt intake increased resting pre-restraint values of MAP by 16±6 mm Hg (P=0.02) in AT1A−/− mice and also tended to increase MAP by 7±4 mm Hg in AT1A+/+ mice (Table 1). Thus, on a high-salt diet, resting MAP in AT1A−/− mice remained lower by 17±5 mm Hg than that in AT1A+/+ mice (P<0.01). Conversely, high salt intake caused little changes in pre-stress values of HR and locomotor activity, which were thus not different between groups (Table 1).
Under conditions of high salt intake, the pressor response to restraint stress in AT1A−/− mice (30±3 mm Hg) was significantly increased (P=0.03) in comparison with that on normal salt intake (20±3 mm Hg; Figure 1), and was no longer different from that in AT1A+/+ mice (28±3 mm Hg; Figure 3). Likewise, the stress-induced tachycardia and locomotor activation did not differ between groups (Figure 3).
(a) Effect of high sodium intake on cardiovascular and locomotor response to restraint stress in AT1A−/− and AT1A+/+ mice. Abbreviations are as in Figure 1. (b) Relationship between MAP and locomotion during the restraint test in AT1A−/− and AT1A+/+ mice.
There was a significant correlation between MAP and locomotion in both AT1A−/− mice (r=0.31, P<0.01) and AT1A+/+ mice (r=0.28, P=0.017) during the test. The AT1A−/− and AT1A+/+ mice had a similar responsiveness of MAP to locomotor activity (4.2±1.1 and 4.9±2.0 mm Hg per log10AU, respectively) during the test (Figure 3).
Effect of high salt intake on response to feeding
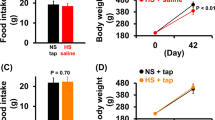
The resting pre-feeding values of MAP were increased on high salt intake by 12±4 mm Hg (P=0.02) in AT1A−/− mice, but not in AT1A+/+ mice (2±4 mm Hg; Table 1). Thus, on a high-salt diet, resting MAP in AT1A−/− mice remained lower by 14±5 mm Hg than that in AT1A+/+ mice (P=0.01). High salt intake did not affect pre-feeding values of HR and locomotor activity, which were thus not different between groups (Table 1).
Under conditions of high salt intake, food presentation and feeding were accompanied by similar increases in MAP in AT1A+/+ and AT1A−/− mice (27±3 and 25±2 mm Hg, respectively; Figure 4). These responses were very similar to those on normal salt intake (26±3 and 25±3 mm Hg, respectively; Figure 2). Likewise, the HR responses to feeding in AT1A+/+ and AT1A−/− mice (131±17 and 134±23 b.p.m., respectively; Figure 4) were not different from those on normal salt intake (Figure 2).
(a) Effect of high sodium intake on cardiovascular and locomotor response to feeding in AT1A−/− and AT1A+/+ mice. Abbreviations are as in Figure 1. (b) Relationship between MAP and locomotion during feeding in AT1A−/− and AT1A+/+ mice.
There was a significant correlation between MAP and locomotion in AT1A−/− mice (r=0.51, P<0.001), but not in AT1A+/+ mice (r=0.16, NS) during the test (Figure 4). The responsiveness of MAP to locomotor activity in AT1A−/− mice on a high-salt diet (5.6±0.8 mm Hg per log10AU) was similar to that on normal salt intake (7.0±0.8 mm Hg per log10AU).
Discussion
This study shows for the first time that targeted deletion of the AT1A receptor gene in mice leads to an attenuation in the pressor response to negative emotional stress. Conversely, AT1A receptor deficiency has little effect on cardiovascular arousal induced by appetitive events, such as presentation and eating palatable food. Another new finding of this study is that AT1A receptor deficiency might promote the sensitivity of blood pressure to synergistic effects of dietary salt and stress. Finally, we report for the first time that there is little interaction between the AT1A receptor, excess dietary sodium and feeding-induced cardiovascular arousal.
Stress response
Pharmacological evidence indicates that AT1 receptors are crucially involved in regulating the cardiovascular response to negative emotional stress.5, 6 In particular, it has been shown that central or systemic administration of AT1 receptor antagonists attenuated the increase in blood pressure and plasma catecholamine levels caused by several psycho- and physico-emotional stressors in rats and rabbits.12, 24, 25, 26 Our data extend these findings by showing that targeted deletion of the AT1A receptor gene selectively attenuates the pressor response to stress in mice. Taken together, the above pharmacological and functional genomic studies strongly suggest that AT1 receptors, and specifically AT1A subtype for murines, are required for full expression of the cardiovascular component of fight-or-flight response.
The precise mechanisms that underlie the attenuation of the pressor response to restraint stress in AT1A−/− mice are yet to be fully clarified. Little effect of AT1A receptor knockout on the pressor response to feeding suggests that the reduction in cardiovascular reactivity could not simply be attributed to changes in basal levels of blood pressure. Given that locomotor activity is an independent determinant of cardiovascular arousal,20, 23 another possibility would be that this attenuation was because of a diminished responsiveness to locomotion during stress. However, AT1A−/− and AT1A+/+ mice have a similar pressor response to a given increase in locomotor activity. Thus, also considering a weak correlation between locomotion and blood pressure during the test, it seems unlikely that the decrease in cardiovascular reactivity in AT1A−/− mice was crucially influenced by locomotion.
It is known that circulating angiotensin II, production of which rapidly increases in response to stress,27 can influence cardiovascular reactivity at the peripheral vascular level by facilitating catecholamine synthesis and release, and/or increasing vascular reactivity to noradrenaline.28, 29 Hence, AT1A receptor deficiency in our study could attenuate the vascular responsiveness to noradrenaline and possibly other circulating stress mediators. Earlier reports, however, suggested that AT1A-receptor gene knockout does not alter the isolated carotid artery responses to several vasoconstrictor agents, including α-adrenoceptor agonist phenylephrine, thromboxane A2 agonist U46619, serotonin and KCl.30 Perhaps more importantly, cardiovascular arousal caused by feeding was not altered in our AT1A−/− mice, further indicating that vascular responsiveness to central pressor stimuli remained essentially intact in these animals. Therefore, it seems unlikely that the decrease in cardiovascular reactivity in AT1A−/− mice was principally mediated by changes in the vascular contractile response to stress-induced sympathetic stimulation.
It is thus conceivable that a disrupted CNS regulation of autonomic outputs to the periphery might underlie the reduction in cardiovascular reactivity in AT1A−/− mice. Our preliminary data showing that aversive (cage-switch) stress-induced expression of a marker of neuronal activation c-Fos protein is attenuated in the DMH and RVLM of AT1A−/− mice by 58 and 42%, respectively,31 are in accord with this notion. Earlier pharmacological studies showing that AT1 receptors in the DMH and RVLM are required for full expression of the pressor response to aversive (air-jet) stress in rabbits12, 26 further support this possibility.
Food presentation and feeding
It is well established that not only aversive, but also appetitive stimuli are capable of inducing a distinct, sympathetically mediated rise in blood pressure in both animals and human participants.8, 9, 10 In particular, food presentation and feeding are normally accompanied in animals by marked increases in blood pressure and heart rate.8, 12, 21 This feeding-associated arousal is initiated by the activation of descending inputs from higher CNS rather than viscero-sympathetic reflexes because of food intake.32, 33 In this study, presentation and eating palatable food elicited distinct pressor and tachycardic responses, which, in wild-type animals, were of similar magnitude to those caused by aversive stimuli. Likewise, we have recently shown that mild aversive (air-jet) and appetitive (straw) stimuli induced very similar cardiovascular arousal in normal rabbits.12 It is intriguing that in contrast to aversive stress, the pressor response to presentation and eating palatable food (almond) was not altered in AT1A−/− mice. This finding indicates that AT1A-receptor gene deficiency does not affect cardiovascular correlates of normal feeding behavior, which is regarded as appetitive or positively motivated.34 These results are in accord with our recent observation that Ren-1c enhancer knockout mice have an attenuated response to restraint and shaker stress, but not to feeding.21 Thus, in relation to cardiovascular reactivity, AT1A−/− mice and Ren-1c enhancer knockout mice show essentially the same phenotype. This observation indicates that the above phenotypes might specifically relate to the dysregulated renin–angiotensin system, and not to the activation of genetic compensatory mechanisms or disruption of neighboring gene expression. In line with this possibility, we earlier reported that pharmacological blockade of AT1 receptors in the DMH (a region crucial for the regulation of autonomic cardiovascular arousal) decreased the pressor response to air-jet stress, but not food presentation and feeding in rabbits.12 Collectively, these data suggest that, with respect to cardiovascular arousal, the brain–angiotensin II system might be primarily involved in regulating the fight-or-flight response, making it a potential therapeutic target for selective attenuation of cardiovascular hyperreactivity to aversive stress.
Effect of high salt intake on stress response
Evidence indicates that high salt intake can reinforce the pressor effect of emotional stress in genetically predisposed animals or human subjects.13, 14, 15 Brain AT1 receptors are likely to play a crucial role in this synergistic effect of salt and stress, because the enhanced pressor and sympathetic responses to air-jet stress could be prevented by central blockade of these receptors in several salt-sensitive animal models, including spontaneously hypertensive and Dahl salt-sensitive rats.16, 17 Importantly, the same treatment regimen had no effect on the stress response in Dahl salt-resistant rats,17 indicating that the aforementioned synergistic effect could be attributed to the salt-induced hyperactivity of central AT1 receptors in susceptible animals.
In this study, a high-salt diet facilitated the pressor response to aversive stress in AT1A−/−, but not AT1A+/+ mice. It is unlikely that this disparity was mediated by the salt-induced change in basal blood pressure and/or vascular reactivity, because the pressor response to feeding was not altered in AT1A−/− mice on high salt intake. It is therefore conceivable that the increase in cardiovascular reactivity in these animals primarily reflected a central synergistic interaction between acute stress and salt. Thus, apart from AT1 receptor hyperactivity,16, 17 genetic deficiency of this receptor might increase the sensitivity of blood pressure to synergistic effects of salt and stress. Further research will be necessary to identify the precise mechanisms that underlie the interplay between the functional state of AT1 receptor and synergistic effect of salt and stress on blood pressure.
Effect of high salt intake on response to feeding
An intriguing new finding of this study is that the salt-induced increase in blood pressure reactivity to negative stress in AT1A−/− mice was not accompanied by a rise in cardiovascular arousal associated with appetitive feeding behavior. This finding indicates that central pressor mechanisms that control feeding-associated arousal are not only independent of AT1A receptors, but also resistant, at least under experimental conditions, to dietary salt loading. Further research is necessary to identify the chemical nature of these mechanisms.
In summary, these results indicate that genetic deficiency of AT1A receptor in mice leads to an attenuated pressor response to negative emotional stress, but not cardiovascular arousal caused by appetitive feeding behavior. The reduction in stress reactivity in AT1A−/− mice seems not to relate to altered locomotion or vascular contractile responsiveness to central pressor stimuli. Our results also indicate that AT1A receptor deficiency is an important factor for the salt-induced augmentation of cardiovascular stress responses. Conversely, there is little relation between the AT1A receptor, excess dietary sodium and cardiovascular arousal associated with feeding. These results might have clinical implications as they suggest that AT1 receptors could be a selective therapeutic target for preventing detrimental synergistic effects of salt and stress on the cardiovascular system.
Conflict of interest
The authors declare no conflict of interest.
References
Esler M . Stress, stressors and cardiovascular disease. Repatriation Medical Authority Consensus Conference on Stress and Challenge—Health and Disease. Repatriation Medical Authority: Brisbane, Australia,, pp. 198–206.
Rozanski A, Blumenthal JA, Kaplan J . Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 1999; 99: 2192–2217.
Hirasawa R, Hashimoto K, Ota Z . Role of central angiotensinergic mechanism in shaking stress-induced ACTH and catecholamine secretion. Brain Res 1990; 533: 1–5.
Kregel KC, Stauss H, Unger T . Modulation of autonomic nervous system adjustments to heat stress by central ANG II receptor antagonism. Am J Physiol 1994; 266: R1985–R1991.
Watanabe T, Fujioka T, Hashimoto M, Nakamura S . Stress and brain angiotensin II receptors. Crit Rev Neurobiol 1998; 12: 305–317.
Saavedra JM, Ando H, Armando I, Baiardi G, Bregonzio C, Jezova M, Zhou J . Brain angiotensin II, an important stress hormone: regulatory sites and therapeutic opportunities. Ann NY Acad Sci 2004; 1018: 76–84.
Baltatu O, Campos LA, Bader M . Genetic targeting of the brain renin-angiotensin system in transgenic rats: impact on stress-induced renin release. Acta Physiol Scand 2004; 181: 579–584.
Del Bo A, Le Doux JE, Reis DJ . Sympathetic nervous system and control of blood pressure during natural behaviour. J Hypertens 1985; 3 (Suppl 3): S105–S106.
Braesicke K, Parkinson JA, Reekie Y, Man MS, Hopewell L, Pears A, Crofts H, Schnell CR, Roberts AC . Autonomic arousal in an appetitive context in primates: a behavioural and neural analysis. Eur J Neurosci 2005; 21: 1733–1740.
Brosschot JF, Thayer JF . Heart rate response is longer after negative emotions than after positive emotions. Int J Psychophysiol 2003; 50: 181–187.
Pressman SD, Cohen S . Does positive affect influence health? Psychol Bull 2005; 131: 925–971.
De Matteo R, Head GA, Mayorov DN . Angiotensin II in dorsomedial hypothalamus modulates cardiovascular arousal caused by stress but not feeding in rabbits. Am J Physiol Regul Integr Comp Physiol 2006; 290: R257–R264.
Folkow B . Critical review of studies on salt and hypertension. Clin Exp Hypertens A 1992; 14: 1–14.
Turkkan JS, Story MK . Blood pressure hyperreactivity in non-human primates during dietary sodium combined with behavioral stress. Integr Physiol Behav Sci 1991; 26: 98–107.
DiBona GF, Jones SY . Analysis of renal sympathetic nerve responses to stress. Hypertension 1995; 25: 531–538.
Huang BS, Leenen FH . Brain ‘ouabain’ and angiotensin II in salt-sensitive hypertension in spontaneously hypertensive rats. Hypertension 1996; 28: 1005–1012.
Huang BS, Leenen FHH . Both brain angiotensin II and ‘ouabain’ contribute to sympathoexcitation and hypertension in Dahl S rats on high salt intake. Hypertension 1998; 32: 1028–1033.
Oliverio MI, Best CF, Kim HS, Arendshorst WJ, Smithies O, Coffman TM . Angiotensin II responses in AT(1A) receptor-deficient mice: a role for AT(1B) receptors in blood pressure regulation. Am J Physiol Renal Physiol 1997; 41: F515–F520.
Chen Y, Joaquim LF, Farah VM, Wichi RB, Fazan Jr R, Salgado HC, Morris M . Cardiovascular autonomic control in mice lacking angiotensin AT1a receptors. Am J Physiol Regul Integr Comp Physiol 2005; 288: R1071–R1077.
Kario K, Schwartz JE, Pickering TG . Ambulatory physical activity as a determinant of diurnal blood pressure variation. Hypertension 1999; 34: 685–691.
Jackson K, Head GA, Morris BJ, Chin-Dusting J, Jones E, La Greca L, Mayorov DN . Reduced cardiovascular reactivity to stress but not feeding in renin enhancer knockout mice. Am J Hypertens 2007; 20: 893–899.
Butz GM, Davisson RL . Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiol Genomics 2001; 5: 89–97.
Leary AC, Donnan PT, MacDonald TM, Murphy MB . The influence of physical activity on the variability of ambulatory blood pressure. Am J Hypertens 2000; 13: 1067–1073.
Saiki Y, Watanabe T, Tan N, Matsuzaki M, Nakamura S . Role of central ANG II receptors in stress-induced cardiovascular and hyperthermic responses in rats. Am J Physiol 1997; 272: R26–R33.
Cierco M, Israel A . Role of angiotensin AT1 receptor in the cardiovascular response to footshock. Eur J Pharmacol 1994; 251: 103–106.
Mayorov DN, Head GA . AT1-receptors in the RVLM mediate pressor responses to emotional stress in rabbits. Hypertension 2003; 41: 1168–1173.
Yang G, Xi ZX, Wan Y, Wang H, Bi G . Changes in circulating and tissue angiotensin II during acute and chronic stress. Biol Signals 1993; 2: 166–172.
Arribas S, Sanchez-Ferrer CF, Peiro C, Ponte A, Salaices M, Marin J . Functional vascular renin-angiotensin system in hypertensive transgenic rats for the mouse renin gene Ren-2. Gen Pharmacol 1994; 25: 1163–1170.
Jezova M, Armando I, Bregonzio C, Yu ZX, Qian S, Ferrans VJ, Imboden H, Saavedra JM . Angiotensin II AT(1) and AT(2) receptors contribute to maintain basal adrenomedullary norepinephrine synthesis and tyrosine hydroxylase transcription. Endocrinology 2003; 144: 2092–2101.
Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD . Angiotensin II-induced vascular dysfunction is mediated by the AT1A receptor in mice. Hypertension 2004; 43: 1074–1079.
Davern P, Chen D, Head GA, Walther T, Mayorov DN . Neuronal activation in the hypothalamus is reduced in AT1A receptor knockout mice following aversive stress. 2006 HBPRCA Presentations. Hypertension 2007; 49: 1465.
Matsukawa K, Ninomiya I . Changes in renal sympathetic nerve activity, heart rate and arterial blood pressure associated with eating in cats. J Physiol 1987; 390: 229–242.
Diamond P, LeBlanc J . Hormonal control of postprandial thermogenesis in dogs. Am J Physiol Endocrinol Metab 1987; 253: E521–E529.
Reynolds SM, Berridge KC . Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste ‘liking’/‘disliking’ reactions, place preference/avoidance, and fear. J Neurosci 2002; 22: 7308–7320.
Acknowledgements
This work was supported by grants from the National Health and Medical Research Council of Australia and the High Blood Pressure Research Foundation of Australia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, D., La Greca, L., Head, G. et al. Blood pressure reactivity to emotional stress is reduced in AT1A-receptor knockout mice on normal, but not high salt intake. Hypertens Res 32, 559–564 (2009). https://doi.org/10.1038/hr.2009.59
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2009.59
Keywords
This article is cited by
-
Role of hypothalamic angiotensin type 1 receptors in pressure overload-induced mineralocorticoid receptor activation and salt-induced sympathoexcitation
Hypertension Research (2013)
-
The Day-Night Difference of Blood Pressure Is Increased in AT1A-Receptor Knockout Mice on a High-Sodium Diet
American Journal of Hypertension (2010)
-
Cardiovascular Responses to Aversive and Nonaversive Stressors in Schlager Genetically Hypertensive Mice
American Journal of Hypertension (2010)