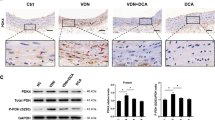
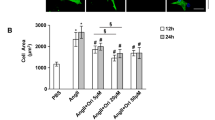
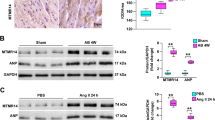
Abstract
It has recently been recognized that adiponectin protects the vasculature and prevents atherosclerotic change through AMP-activated protein kinase (AMPK) activation, and some of its molecular mechanisms have been clarified. AMPK, which might be a therapeutic target of metabolic abnormality, is a serine-threonine kinase, heterotrimer protein composed of three subunits of α, β and γ. It is activated by an upper kinase LKB1 and an increase in the AMP/ATP ratio. Some anabolic enzymes are directly phosphorylated and inhibited, suggesting that AMPK suppresses ATP consumption by negatively regulating the synthetic pathway. The LKB1–AMPK pathway is pivotal for controlling cellular polarity and mitosis. Furthermore, AMPK has been associated with cellular autophagy. AMPK activation could induce autophagy and prolong a period leading to cell apoptosis. Apoptosis under anoxic conditions was decreased when newly constructed, constitutively active mutants of AMPK-α were overexpressed in vascular endothelial cells. AMPK could inhibit the growth of vascular smooth muscle through MEK–ERK pathway inhibition. After ischemia reperfusion, dominant-negative AMPK overexpression inhibits cardiac function through the suppression of glucose uptake and fatty acid β-oxidation in cardiac myocytes. Cardiac hypertrophy with accumulation of glycogen granules because of gene mutation of γ2 associated with the Wolff-Parkinson-White syndrome has been considered an activated type in most cases. It is necessary to clarify the tissue-specific and stress-specific activation mechanism of AMPK.
Similar content being viewed by others
Introduction
For blood vessels exposed to physical stress, such as blood pressure or shear stress, and for various bioactive substances to maintain homeostasis, it is necessary to reduce stresses and, in such cases, the vascular endothelium needs to act against arterial sclerosis. In addition, the control of growth and apoptosis in the neointima of vascular smooth muscles is important for the development of an arterial sclerosis lesion.
Conversely, the recent progress in adiposcience with regard to metabolic syndrome has revealed the cardiovascular protective function of so-called ‘beneficial’ adipocytokines. A part of the action mechanism of adiponectin, a representative of adipocytokines, is recognized to be caused by AMP-activated protein kinase (AMPK),1, 2 and a part of its molecular mechanisms has been clarified. It has also been reported that some diabetes remedies, such as metformin or thiazolidine derivates, can activate AMPK,3, 4, 5 suggesting the possibility that AMPK can become a therapeutic target of metabolic abnormalities.
In this review, the recently clarified action of AMPK is summarized and its functions in the cardiovascular system are described.
The structure and regulatory mechanism of AMPK
Overview
The AMP-activated protein kinase is a serine-threonine kinase cloned as a homolog of the Snf1 kinase of yeast and is a heterotrimer protein composed of three subunits, α, β and γ6 (Figure 1). Generally, kinase activity is increased by stress, which elevates AMP/ATP in cases such as hypoxia, low glucose and contractions/exercises of the skeletal muscle.
Recently, activation mechanisms other than the elevation of the AMP/ATP ratio (for example, reactive oxygen species (ROS)) have garnered increasing attention. The factors activating AMPK are listed in Table 1. In mammals, there are two types of α and β subunits and three kinds of γ subunits. The correspondence with the Snf1 kinase family is shown in Table 2. It has also been revealed that there is a difference in the distribution of these subunits depending on the tissue (Figure 1).
α-Subunit
This is the essential part of AMPK with kinase activity. In the case of humans, α1 is composed of 550 amino acids and α2 of 552 amino acids. The kinase domain is on the N-terminus of the α-subunit. There are β-subunit-binding domains on the C-terminus (Figure 1). If α Thr172 in the kinase domain is phosphorylated by an upstream kinase (AMPK kinase, AMPKK), kinase activity is increased dozens of times7 (Figures 2 and 3). When it is inactivated adversely, the phosphorylation domain is dephosphorylated by protein phosphatase-2C.8 In the α-subunit, there is an autoinhibitory domain that inhibits its kinase activation (Figure 2). It is thought that in the cardiovascular system (in particular, the heart), primarily α2 is expressed, whereas in the vascular endothelial cell, α1 is more abundantly expressed.
Activation mechanism of AMP-activated protein kinase (AMPK) (a, b). If γ binds to ATP, it enters a locked state, but binding to AMP unlocks it. When α Thr172 is phosphorylated by an upstream kinase, such as LKB1, inhibition in the autoinhibitory domain is released. The AMPK trimer complex exerts full kinase activity. AID, autoinhibitory domain; K, kinase domain.
β-Subunit
The β-subunit is considered to have a role in the platform of α and γ binding. α and γ bind to kinase interacting sequence (KIS) and association with Snf1p complex (ASC) domains (Figure 1). Furthermore, it has been noted that the N-terminus is myristorylated, which may determine substrate specificity, but the details are unclear.
γ-Subunit
The γ-subunit has the configuration of a line of four cystathionine β-synthase (CBS) domains having avidity with AMP/ATP, and two CBS domains exist in tandem to form the basic functional unit (Figure 1), called the Bateman domain.
On the basis of current knowledge, the schema illustrating how the α, β and γ subunits are actually bound and act is shown in Figure 2. When AMP is bound in place of ATP, the locking function of γ is unlocked, α Thr172 is phosphorylated by an upstream kinase and inhibition in the autoinhibitory domain is released, which activates the kinase activity of AMPK.
On the other hand, a recent paper describes γ-subunit binding to the β-subunit through the α-subunit; the γ-subunit does not seem to bind directly to the β-subunit.9 However, there have been no additional tests confirming these results.
AMPKK
Although the actual property of an upstream kinase, AMPKK, has remained unclear, it is suggested that LKB1, the responsible kinase for Peutz-Jeghers syndrome, is the most important AMPKK.10, 11 It is also known that LKB1 is activated by binding to both mouse protein 25 (MO25) and STE20-related adaptor protein (STRAD), which are adaptor proteins.11 On the other hand, it was previously considered that AMPKK is a serine-threonine kinase activated by AMP, but it was clarified that LKB1 is not activated by AMP.11, 12
Furthermore, there is a report describing that, in some cases, AMPK is not activated by AMP/ATP,3 suggesting that, other than LKB1, there may be AMPKK, which can activate AMPK without depending on AMP/ATP. In fact, multiple groups have revealed that its entity is Ca2+/calmodulin-dependent protein kinase kinase, which has a higher homology with LKB1.13, 14
Functions of AMPK
Functions at cellular levels
In various types of cells, when AMPK is activated, translocation of GLUT-4 to the plasma membrane is accelerated and the uptake of glucose into cells is increased. In addition, 3-hydroxy-3-methylglutaryl-CoA reductase or acetyl-coA carboxylase is directly phosphorylated to inhibit enzyme activity and control ATP consumption by negatively regulating the synthetic pathway (Table 3). This is why AMPK is described as a ‘fuel gauge’ within the cell. Figure 4 illustrates the mechanisms for enhancing carnitine palmitoyltransferase-1 (CPT-1) activity and promoting β-oxidation of fatty acids in the mitochondria. It has also been shown that AMPK increases mitochondrial biosynthesis through synthesis increases in some mitochondrial-constitutive enzymes.15
The mechanism of fatty acid β-oxidation enhancement by AMP-activated protein kinase (AMPK). AMPK directly phosphorylates acetyl-CoA carboxylase 2 (ACC2), inhibits enzymatic activity and inhibits synthesis of malonyl-CoA. In turn, fat synthesis is inhibited, and fatty acid β-oxidation is enhanced through increased activity of carnitine palmitoyltransferase-1 (CPT-1).
In addition, the regulating mechanism of the mTOR/S6K system by AMPK has recently been clarified. Activated AMPK inhibits mammalian target of rapamycin (mTOR) by activating the tuberous sclerosis complex through phosphorylation and decreases protein synthesis through the action of (p70)S6K and 4EBP-1 further downstream; it also inhibits cell growth and hypertrophy16 (Figure 5). Akt shows just the reverse action from that of AMPK because it inhibits by phosphorylating another domain of tuberous sclerosis complex.
Protein synthesis inhibiting the action of AMP-activated protein kinase (AMPK) through tuberous sclerosis complex 2 (TSC2). The functional directions of AMPK and Akt on which the pathway for protein synthesis through mammalian target of rapamycin (mTOR)/S6K might act are opposing. AMPK increases TSC1-TSC2 activity, whereas Akt inhibits it.
Control of cell polarity/cell mitosis
It has been observed that in Caenorhabditis elegans, PAR4, which is a kinase homogenous with LKB1, is needed to determine cephalocaudal polarity.17 In Drosophila, it was shown that LKB1 is needed for the determination of anterior–posterior polarity of the oocyte,18 and in fact, it was clarified that even in mammals this system is important for controlling polarity in intestinal epithelial cells.19 Furthermore, a similar phenomenon has been reported in the downstream AMPK. It is possible to generate Drosophila completely lacking in AMPK, but such cases become lethal in their development pathway. The polarity of the cell or mitosis is not normally controlled, and regularity in the actin cytoskeleton in endothelial cells is lost. In the case of a complete defect of LKB1, a similar phenotype is observed, but it has been found that the above-mentioned abnormality can be restored by making constitutively active mutants of AMPK expressed in the LKB1 deletion form.20 Furthermore, the myosin regulatory light chain is important for controlling cellular polarity and mitosis. It has been shown that this protein is phosphorylated by AMPK and controls the interaction between nonmuscle myosin and actin and contributes to the above-mentioned aberration (in mammals, Drosophila, and so on).20 In addition to its function in the control of polarity, myosin regulatory light chain has also been found in MDCK cells derived from epithelial cells from the collecting tube of dogs; assembly and disassembly of the intercellular tight junction are controlled by AMPK.21 Many papers, including the above-mentioned reports, suggest that the LKB1–AMPK system has an important role in controlling cellular polarity and mitosis.
The control of autophagy
Recently, there have been several reports describing how AMPK is associated with the control of cellular autophagy. Autophagy is, in brief, the mechanism by which the cell produces energy using subcellular organelles or proteins in an autologous cell as the substrate; it enables survival when the cell is exposed to crises such as alimentary deficiency.22 AMPK is activated in the cardiac myocyte through hypoglycemia and hypoxia (ischemia), but a system to induce autophagy through inhibition of mTOR and avoid cellular death has been reported.23 In the report, the activation of AMPK using 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) failed to induce autophagy; thus, activation of AMPK may not be a sufficient condition for autophagy to occur. On the other hand, a review of human mammary cancer-derived cells (MCF-7s) revealed that the apoptosis occurring in serum depletion is inhibited when p27kip Thr198 is phosphorylated by AMPK; p27kip is not broken down under such conditions, and autophagy occurs.24 When a phosphorylation domain of p27kip is replaced by alanine or the expression amount is reduced by p27kip small interfering RNA, autophagy is inhibited and apoptosis occurs efficiently. That is, the amount of p27kip determines whether apoptosis or autophagy is initiated, and apoptosis is avoided to some extent. It seems that AMPK can induce autophagy and prolong the period leading to cell death.
Function at an individual level
At the cellular level, AMPK increases ATP levels, but, even in a living organism, it increases appetite and energy. In other words, in the hypothalamus, ghrelin and fasting activate AMPK, which increases the expression of NPY and elevates appetite. Leptin, insulin and diet adversely suppress hypothalamic AMPK, reduce the expression of NPY and inhibit appetite (Figure 6)7. The details of the NPY regulation mechanism by AMPK are still unclear.
Function in the cardiovascular system
The AMP-activated protein kinase is one of the key molecules linking energy metabolism and atherosclerotic change in vasculature. In this paper, we describe the functions of AMPK in the cardiovascular system.
Function in the heart
In the heart, AMPK has an effect even in the physiological state,25 and the importance of the action of AMPK is increased under conditions in which stress, such as excessive load or ischemia, is induced under hypoxic conditions. AMPK activates the glycolytic pathway by phosphorylating and activating phosphofructokinase-2,26 enhancing fatty acid β-oxidation and ameliorating relative ATP deficiencies.27 Furthermore, it promotes the translocation of GLUT-4 to the plasma membrane and increases the uptake of glucose to the skeletal muscle.28 In a transgenic mouse in which dominant-negative AMPK α2 was overexpressed in the cardiac myocyte, glucose uptake after ischemia reperfusion and fatty acid β-oxidation were decreased, causing a decrease in ATP, and cardiac function was significantly lower when compared with that of the control; furthermore, myocardial apoptosis was increased.29 One report indicated that a partial cause of the significant increase in myocardial apoptosis after ischemia reperfusion in the adiponectin knockout mouse is associated with AMPK activation deficiency due to deficits of adiponectin.30
Recent reports described a family whose members developed cardiac hypertrophy with glycogen granule accumulation in the cardiac myocyte associated with Wolff-Parkinson-White syndrome because of an aberration in γ2 genes.31, 32 On the other hand, with regard to mutations (for example, Arg302Gln) of γ in the same domain, one report described it as an activated type32 and the another as a nonactivated type.33 However, cardiac hypertrophy with an accumulation of glycogen granules because of a gene mutation of γ2 is considered as the activated type in most cases. The review by Arad et al.34 summarizes these details effectively. When AMPK is activated, glycogen synthase activity is directly inhibited (Table 3), but it has been thought that the fatty acid oxidation promoted by AMPK enables ATP supply; thus, intracellular glucose is not used, and glycogen is thought to accumulate in the cardiac myocyte.
Function in the vasculature
Vascular endothelium
It is thought that the vascular endothelial cell alleviates the action of tissue injury factors such as oxidative stress and ameliorates arteriosclerotic change at early stages. In particular, endothelial nitric oxide synthase (eNOS) is important for maintaining endothelial function through the production of NO. We have reported that AMPK activated under hypoxic conditions maintains Akt activity and activates eNOS through Ser1177 phosphorylation.35, 36 This action suggests that NO production is important for angiogenesis under hypoxic conditions. Another group reported that in the endothelium, adiponectin activates AMPK and activates eNOS through the PI3K–Akt system.37 Another report described how AMPK activation contributes to eNOS activation by high-density lipoprotein and apolipoprotein AI, which is the constituting apolipoprotein of high-density lipoprotein.38 In addition, an interesting report described how AMPK activated by metformin inhibits the nuclear factor-κB signal increased by cytokine stimulation;4 the detailed mechanism is reviewed hereafter.
On the other hand, Ido et al.39 reported that hyperglycemia induces apoptosis through oxidative stress, but AMPK activated by AICAR partially inhibits this phenomenon. One report described how adiponectin inhibits apoptosis in the endothelial cell through AMPK activation.40 We recently made an AMPK construct lacking only the autoinhibitory domain (amino acids 313–392) of AMPKα1; the binding capacity to the β-subunit remains, and Thr 172 is substituted by Asp, enabling overexpression of the constitutively active form.36 This system allows the observation of the specific action of AMPK without the nonspecific action of AICAR. Apoptosis under anoxic conditions was decreased significantly when we overexpressed this mutant in vascular endothelial cells.36 As mentioned above, in the endothelial cell, AMPK activates the PI3K–Akt system, and it has been shown that this antiapoptotic effect takes place through the PI3K–Akt axis.41
The vascular smooth muscle
We have shown that the growth of the vascular smooth muscle cell stimulated by angiotensin II (AngII) can be inhibited by the activation of AMPK.42 When we tried to stimulate cells by AngII, to our surprise, AMPK was activated, and it depended on ROS through the AngII type I receptor. On the other hand, when AMPK is further activated by AICAR, the growth of vascular smooth muscle was inhibited, and it became clear that inhibition of the MAP kinase system was pivotal for this antiproliferative action. In the wire injury model of the rat femoral artery, if AMPK is continuously activated by AICAR injection, neointima formation is significantly inhibited, and AMPK inhibits the growth of the vascular smooth muscle, even in vivo. Igata et al.41 revealed that AMPK increases p21cip, which is the cyclin-dependent kinase inhibitor, through inhibition of the phosphorylation of the retinoblastoma gene product (Rb), as well as increased expression/phosphorylation enhancement of p53, and inhibits the cell cycle of the vascular smooth muscle at G1/S. Recently, the presence of a mechanism has shown that the activation of thromboxane A2 receptor promotes hypertrophy of the vascular smooth muscle and activates AMPK ROS dependently. The activation of AMPK inhibits hypertrophy of the vascular smooth muscle cell.43 This negative feedback system is very similar to that which we have reported in the AngII type I receptor.
Conclusion
It is certain that AMPK is a kinase highly conserved from yeasts to humans, and it performs an important action for organisms under conditions of stress. Hereafter, it is necessary to clarify the tissue-specific and stress-specific activation mechanism. Currently, there are many unclarified details with regard to, for example, why the action is different in a cell-specific manner, as shown by the fact that AMPK activates PI3K–Akt in vascular endothelial cells, whereas in other cells, the actions of AMPK and Akt are frequently antagonistic to one another. This underscores the importance of reexamining the pathophysiological actions of AMPK.
References
Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T . Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002; 8: 1288–1295.
Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB . Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 2002; 99: 16309–16313.
Fryer LG, Parbu-Patel A, Carling D . The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem 2002; 277: 25226–25232.
Hattori Y, Suzuki K, Hattori S, Kasai K . Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 2006; 47: 1183–1188.
Konrad D, Rudich A, Bilan PJ, Patel N, Richardson C, Witters LA, Klip A . Troglitazone causes acute mitochondrial membrane depolarisation and an AMPK-mediated increase in glucose phosphorylation in muscle cells. Diabetologia 2005; 48: 954–966.
Hardie DG . Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 2003; 144: 5179–5183.
Hardie DG . New roles for the LKB1 → AMPK pathway. Curr Opin Cell Biol 2005; 17: 167–173.
Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Gorgun CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE . Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab 2006; 4: 465–474.
Wong KA, Lodish HF . A revised model for AMP-activated protein kinase structure: the alpha-subunit binds to both the beta- and gamma-subunits although there is no direct binding between the beta- and gamma-subunits. J Biol Chem 2006; 281: 36434–36442.
Hong SP, Leiper FC, Woods A, Carling D, Carlson M . Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc Natl Acad Sci USA 2003; 100: 8839–8843.
Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG . Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2003; 2: 28.
Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D . LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 2003; 13: 2004–2008.
Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG . Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2005; 2: 9–19.
Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D . Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2005; 2: 21–33.
Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI . AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA 2002; 99: 15983–15987.
Inoki K, Zhu T, Guan KL . TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003; 115: 577–590.
Watts JL, Morton DG, Bestman J, Kemphues KJ . The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development 2000; 127: 1467–1475.
Martin SG, St Johnston D . A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature 2003; 421: 379–384.
Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC . Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 2004; 116: 457–466.
Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, Chung J . Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 2007; 447: 1017–1020.
Zheng B, Cantley LC . Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci USA 2007; 104: 819–822.
Levine B, Klionsky DJ . Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 2004; 6: 463–477.
Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J . Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 2007; 100: 914–922.
Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, Slingerland JM, Mills GB . The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 2007; 9: 218–224.
Makinde AO, Gamble J, Lopaschuk GD . Upregulation of 5′-AMP-activated protein kinase is responsible for the increase in myocardial fatty acid oxidation rates following birth in the newborn rabbit. Circ Res 1997; 80: 482–489.
Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L . Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol 2000; 10: 1247–1255.
Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD . High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem 1995; 270: 17513–17520.
Russell III RR, Bergeron R, Shulman GI, Young LH . Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol 1999; 277: H643–H649.
Russell III RR, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH . AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest 2004; 114: 495–503.
Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K . Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 2005; 11: 1096–1103.
Gollob MH, Green MS, Tang AS, Gollob T, Karibe A, Ali Hassan AS, Ahmad F, Lozado R, Shah G, Fananapazir L, Bachinski LL, Roberts R . Identification of a gene responsible for familial Wolff-Parkinson-White syndrome. N Engl J Med 2001; 344: 1823–1831.
Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, McGarry K, Seidman JG, Seidman CE . Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest 2002; 109: 357–362.
Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG . CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest 2004; 113: 274–284.
Arad M, Seidman CE, Seidman JG . AMP-activated protein kinase in the heart: role during health and disease. Circ Res 2007; 100: 474–488.
Nagata D, Mogi M, Walsh K . AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem 2003; 278: 31000–31006.
Nagata D, Kiyosue A, Takahashi M, Satonaka H, Tanaka K, Sata M, Nagano T, Nagai R, Hirata Y . A new constitutively active mutant of AMP-activated protein kinase inhibits anoxia-induced apoptosis of vascular endothelial cell. Hypertens Res 2009; 32: 133–139.
Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K . Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem 2004; 279: 1304–1309.
Drew BG, Fidge NH, Gallon-Beaumier G, Kemp BE, Kingwell BA . High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. Proc Natl Acad Sci USA 2004; 101: 6999–7004.
Ido Y, Carling D, Ruderman N . Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes 2002; 51: 159–167.
Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y . Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res 2004; 94: e27–e31.
Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, Matsumoto K, Toyonaga T, Asano T, Nishikawa T, Araki E . Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res 2005; 97: 837–844.
Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, Hirata Y . AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation 2004; 110: 444–451.
Zhang M, Dong Y, Xu J, Xie Z, Wu Y, Song P, Guzman M, Wu J, Zou MH . Thromboxane receptor activates the AMP-activated protein kinase in vascular smooth muscle cells via hydrogen peroxide. Circ Res 2008; 102: 328–337.
Acknowledgements
This study was supported by Grants-in-Aid #19590855 (to DN) and #17659229 (to YH), Core Research for Evolutional Science and Technology (to YH) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, Sankyo Foundation of Life Science (to DN) and by the Research Grant for Cardiovascular Diseases (20C–3) from the Ministry of Health, Labour and Welfare of Japan (to DN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagata, D., Hirata, Y. The role of AMP-activated protein kinase in the cardiovascular system. Hypertens Res 33, 22–28 (2010). https://doi.org/10.1038/hr.2009.187
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2009.187
Keywords
This article is cited by
-
MicroRNA Let-7 targets AMPK and impairs hepatic lipid metabolism in offspring of maternal obese pregnancies
Scientific Reports (2021)
-
Perivascular adipose tissue (PVAT) in atherosclerosis: a double-edged sword
Cardiovascular Diabetology (2018)
-
Interactome analysis of AMP-activated protein kinase (AMPK)-α1 and -β1 in INS-1 pancreatic beta-cells by affinity purification-mass spectrometry
Scientific Reports (2014)
-
AMPK in cardiovascular health and disease
Acta Pharmacologica Sinica (2010)