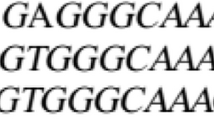Abstract
A mutant Thr-239-Ileu at the α2-tubulin gene was found to confer resistance to dinitroanilines, a family of mitosis-disrupting herbicides. However, mutations affecting microtubule polymerization and cell division are expected to impact growth and reproduction, that is, the fitness of a resistant weed or the yield of a tolerant crop, although it has not been demonstrated yet. This study was designed to test this hypothesis for the growth and reproduction of near-isogenic resistant and susceptible materials that were created in F2 and F3 generations after a Setaria viridis x S. italica cross. Differential growth was noticeable at the very onset of seedling growth. The homozygous resistant plants, grown both in a greenhouse cabinet and in the field, were smaller and had lower 1000-grain weight and therefore a lower yield. This fitness penalty is certainly due to modified cell division kinetics. Although the presence of the mutant allele accounted for 20% yield losses, there were also measurable benefits of dinitroaniline resistance, and these benefits are discussed.
Similar content being viewed by others
Introduction
In addition to the genetically engineered crops that now cover hundreds of millions of hectares, several commercialized herbicide-resistant varieties have been generated through ‘conventional’ methods (Duke, 1996). With the exception of a few cases of gene amplification (Caretto et al., 1994), they originated in mutant germplasms obtained through tissue culture selection, seed or microspore mutagenesis, and even from wild relatives (Tan et al., 2005). Nothing is known about the side effects of these genes in crops. Some insight can be gleaned from the incidence of genes captured from wild relatives; they are often at very low frequency in natural and weed populations, indicating that they suffer some fitness cost in field conditions (see Vila-Aiub et al., 2009 for review of fitness costs linked to herbicide resistance in weeds). When expressed in crops, there could be some yield costs to these modifications that would offset the obvious benefits, such as being able to use appropriate herbicides and to ensure weed-free crop growth. The production of novel or altered proteins could hinder overall metabolism or have pleiotropic effects. For instance, a yield penalty has clearly been observed with triazine-resistant lines of oilseed rape and foxtail millet, with a 19–25% reduction in grain yield when compared with isogenic-susceptible lines grown without the use of herbicide (Beversdorf et al., 1988; Darmency and Pernès, 1989). In contrast, no difference was found between Acetolactate Synthase (ALS)-inhibitor (imidazolinone)-resistant and near-isogenic susceptible lines of maize and sunflower (Boerboom and Lauer, 1997; Massinga et al., 2005). In Arabidopsis thaliana, the difference depended on nutriment availability (Purrington and Bergelson, 1997). The yield penalty question does not appear to be of great general concern, as research into this question has not been yet been reported in the academic literature for commercialized varieties of wheat, oilseed rape, rice, lentil or flax that possess the same ALS resistance mutation (Tan et al., 2005). Therefore, it is not clear whether herbicide resistance genes always have negative pleiotropic effects or whether herbicide-resistant mutated lines always have lower yield potential.
In this paper, we focus on an α2-tubulin mutant gene that confers resistance to dinitroaniline herbicides. It is a good candidate for creating new herbicide-resistant germplasm in cereals. Attempts to improve maize using this mutant gene have been performed through classical breeding (Landi et al., 1999) and through genetic engineering (Anthony and Hussey, 1999). Development of trifluralin-resistant transgenic material has also been proposed, both to improve weed control in the fields and to use as a marker gene (Anthony et al., 1999; Yemets and Blume, 2007). Trifluralin, a mitosis-disrupting herbicide belonging to the dinitroaniline family, has been used widely in cotton, soybeans, oilseed and wheat since the 1970s (Ashton and Crafts, 1981). A total of 10 weed species have evolved trifluralin resistance in response to repeated use of these compounds in the fields. These are primarily grass species, along with two rare dicotyledonous species (Heap, 2010). Fitness costs are expected because any mutation on tubulin genes could affect microtubule polymerization and function, and microtubules have a key role in cell replication. Such a feature has been observed for many mutations that confer dinitroaniline resistance in Protozoa (Ma et al., 2007).
Few studies have been carried out to investigate the fitness consequences of dinitroaniline resistance in weeds, and none have involved isogenic plant material. In goosegrass (Eleusine indica), there were no clear-cut differences between the two types in transplant experiments, although the resistant type often had lower inflorescence dry weight than the susceptible type (Murphy et al., 1986), especially under competitive conditions (Valverde et al., 1988; Harris et al., 1995). In annual bluegrass (Poa annua), resistant plants had lower vegetative growth but there was no consistent difference in reproduction between the two types (Lowe et al., 2001). Finally, in green foxtail (Setaria viridis), high persistence of the trifluralin-resistant type in non-treated fields over several years could support that the two types of this species have equivalent fitness (Andrews and Morrison, 1997).
We crossed resistant green foxtail plants with foxtail millet to obtain a trifluralin-resistant germplasm (Wang et al., 1996). Herbicide-resistant foxtail millet, Setaria italica (L.) Beauv., an autogamous small grain C4 cereal, was in demand because of the lack of selective herbicides and because hand weeding had become impractical for farmers. The mutation responsible for resistance was later identified as a threonine/isoleucine at residue 239 of the α2-tubulin gene (Délye et al., 2004). Here, we report on the impact of this α2-tubulin mutant gene on foxtail millet yield. For the first time, near-isogenic plants were used and they showed an important yield penalty. The near-isogenic material was obtained in the segregating F2 of the initial crosses and F3 generation of a subsequent selection and breeding scheme. In each generation, we compared resistant and susceptible plants that, on average, shared the same genetic background, with the exception of the group of linkages in which the α2-tubulin gene is located. We compared plants using field experiments and a growth cabinet experiment designed to check fine differences at the early seedling stage.
Materials and methods
Plant material
Plants from the ‘Oak River’ population of Setaria viridis (L.) Beauv. that had evolved resistance to a dinitroaniline herbicide (trifluralin) in Canada (Morrison et al., 1989) were crossed to the Amende-4 cultivar of S. italica (L.) Beauv., a spring cv. from Heilongjiang, China (Wang et al., 1996). Resistance was conferred by a single-gene recessive mutation in the wild parent (Jasieniuk et al., 1994). Some F2 seeds collected on the F1 were used in the garden experiment (indicated in boxes in Figure 1). Other F2 seeds were subjected to herbicide treatment (see below) to select homozygous resistant F2 plants. They were subsequently self-pollinated, and their progeny were selected for growth habit and ear morphology corresponding to that of the crop parent (Amende-4) over two generations. Subsequent F4 plants showed little variability, and were used as pollen donors in a cross with one of the most popular cv. in China, Jigu 11, a summer cv. from Hebei. F2 grains from this last cross were germinated on a trifluralin medium (see below) to select the resistant homozygous rr seedlings. At the same time, other F2 grains were germinated on water, and therefore contained homozygous SS and rr as well as heterozygous Sr genotypes. Some 200 seedlings of the two treatments were transferred to Jiffy 7 peat pellets, planted in the garden at Dijon, bagged and individually harvested. The herbicide resistance test was carried out on the F3 grains to identify the genotype of every maternal F2 plant and to select the homozygous rr and SS F3 genotypes. Then, the F3 grains were bulked according to their genotype (indicated in boxes in Figure 1).
SS, Sr and rr identification
The genotype was identified following harvest by characterization of the progeny. A total of 50 grains of each ear were germinated in a petridish on filter paper moistened with commercial trifluralin solution at 0.2 μM active ingredient (Callifort, Calliope, France: 480 g l−1 trifluralin). Seedlings were grown for 3 days in the dark at 25 °C, then classified as susceptible or resistant based on the shoot length (<or>1.5 cm) and the look (swollen or not) of the shoot tips (Wang et al., 1996). Progeny of heterozygous plants produced about 17% viable seedlings instead of the 25% expected for a single-gene recessive mutation because of a distorted segregation ratio (Tian et al., 2006).
F2 garden experiment
A total of 200 F2 seeds of the initial interspecific cross were germinated on moistened paper in a petridish, grown for 3 days at 25 °C, then transferred to Jiffy 7 peat pellets and grown in a growth cabinet at 25 °C under a 16 h photoperiod. Three-leaf seedlings were planted every 8 cm on a row in the experimental garden. The flowering date was recorded when an ear emerged from the main stem. The following measurements were taken at maturity: the height to the last leaf node, the flag leaf width, the number of ears, the length of the main ear, the seed weight of the main ear and the 1000-grain weight. The number of seeds on the main ear was estimated for each plant. Data were analyzed with the GLM procedure of SYSTAT software version 10 (SPSS Inc., Chicago, IL, USA, 1999) after log transformation of the ear length and seed weight on the main ear and seed number.
Growth cabinet experiment
Early growth differences between the genotypes could be reduced by using transplanted seedlings grown in the greenhouse, as for the previous experiment, thus mitigating any differential growth at emergence and at the very earliest stage of plant life. To determine whether this potential bias exists, we investigated the emergence and growth of young seedlings under controlled conditions. A total of 10 rr and 10 SS F3 grains were separately sown in 8 cm2 pots filled with 200 g of a sandy clay soil. The seedlings were grown for 7 days in a growth cabinet over a 16 h photoperiod (200 μM−2 s−1) at 27 °C in the day and 22 °C over night. The emergence rate was scored at the end of the growth period and the emerged seedlings were randomly thinned to either one or three per pot, creating two levels of plant density and generating different levels of competition. Both low- and high-density pots, containing either rr or SS plants, were placed in three growth cabinets. The pots were given 22 ml of water and their positions were randomly changed within a density group every other day. The plant height at the last visible leaf/stem angle was measured the very day a new leaf/stem angle was visible. The plant height, the number of visible leaves and the dry aboveground biomass were measured 38 days after sowing (DAS). Regressions of plant height against leaf range were computed using block values. The data were analyzed with a fixed-effects model analysis of variance for a split-plot design, including three blocks (growth cabinets), two densities (main plots) and two genotypes.
F3 field experiment
F3 grains were sown in a randomized split-plot arrangement at two densities, 20 and 50 plants per m2, corresponding to densities of foxtail millet production in France and China, respectively. There was 0.75 and 0.37 m interrow in the two density treatments, respectively, and five replicate blocks. Within the main plots for each density, there were pure rr and SS and mixed rr/SS subplots. Each subplot consisted of three, 5 m long rows. For the pure plantings, 300 grains were planted per row, and emerged seedlings were counted before being thinned to the desired density (that is, 75 and 92 seedlings per row for the 20 and 50 plants per m2, respectively). For the mixed planting, pinches of five grains of the rr and SS genotype were alternately sown along each row every 7 or 5 cm according to the density. Thinning was done to ensure that only one seedling grew at each planting site.
Measurements were carried out on six plants of the central row (or six rr and six SS for the mixed planting) to avoid any border effect. Height to the last leaf node was measured, and leaf and tiller numbers were counted at two dates (54 DAS and at maturity) to detect any differential growth kinetics. The flowering date was also recorded. Flag leaf length and width, and aboveground dry biomass (excluding ears) were measured at maturity. The reproductive traits measured were the grain number and grain weight both on the main ear and for the overall plant. We also included measurements of the main ear morphology and the 1000-grain weight to describe components of reproductive allocation. The number of grains produced per unit of dry biomass was calculated for every plant. Average data for the six plants measured per treatment and replicate were analyzed using a fixed-effects analysis of variance for a split-plot design that included five blocks, densities (D) as main plots, and genotypes (G) and planting (P) factors as subplots, with G × D, G × P, D × P and G × D × P interactions. We did not detect heterogeneity of variance using Levene's test, thus justifying the use of untransformed data.
Results
F2 garden experiment
The germination rate was 75.5%, 151 seedlings were transplanted and 148 produced seeds. In all, 25% unviable seeds are not unexpected following interspecific crosses. The proportions of rr, Sr and SS genotypes were identified afterward by their progeny: 11.5, 42.6 and 46.9%, respectively. These proportions are consistent with the distorted segregation ratio previously observed (Tian et al., 2006). The Sr and SS genotypes did not differ in the traits measured. The rr genotype had smaller flag leaf width, lower weight of the seeds on the main ear and lower 1000-grain weight than the two other genotypes, but had more tillers (not shown) and secondary ears (Table 1). Owing to high variability, the data for the main ear length and the grain number were highly variable and only marginally significant (P=0.07) but again, the rr genotype exhibited the lowest values.
Growth cabinet experiment
The grains collected on the rr F2 plants weighed less than those collected on the SS plants, 2.67±0.39 and 2.86±0.22 g, respectively (t=4.25, P<0.001). Germination in pots was not different for rr and SS grains: 80±9 and 83±7%, respectively. Thirty-eight DAS results were as follows: rr plants were smaller than SS plants regardless of the density, only the rr plants in high-density pots had fewer leaves and there was no difference in plant weight among treatments (Table 2). High plant density resulted in lower plant height, number of leaves and weight than low density. However, these differences were only observed when morphological data were expressed in terms of growing days. The regression of plant height against leaf range best fit a power law y=xa. The slopes were consistent across plant densities and genotypes (Table 2 and Figure 2).
F3 field experiment
Seedling emergence was not significantly different between rr and SS, 53.9±3.9 and 59.0±2.2%, respectively (t=1.13, P>0.05). The density effect was significant for half of the traits measured (Table 1). Higher plant density resulted in higher leaf number and taller plants 54 DAS, but lower per plant biomass (21.1±1.5 versus 16.6±1.1 g) and grain weight (22.8±2.1 versus 15.3±1.1 g). No genotype interaction was detected. Interestingly, there was no density effect on secondary tiller production, 1000-grain weight or the number of grains per unit of biomass, indicating low plasticity and strict genetic control of these traits. The planting type (pure or mixed) affected the number of leaves and the plant height 54 DAS. There was a significant interaction between the planting type and the genotype for flowering time only, indicating plant competition at the early growth stage.
The genotype effect was significant for 6 of the 20 characters (Table 1). The early records at 54 DAS showed more rapid development of the tillers in the rr plants than those of the SS plants. The rr plants were smaller and had fewer leaves than the SS plants at maturity, but the final number of tillers was similar for both genotypes. There was no difference in the morphology of the flag leaf or the ear. The main ear weight and the 1000-grain weight of the rr plants were lower than the SS plants, whereas the difference in total grain number per plant and grain weight between plants was marginally significant (P=0.08 and 0.09, respectively). Most trait values, except tiller number 54 DAS, were lowest in rr plants.
Discussion
This is the first study dealing with the impact of dinitroaniline resistance on the fitness of near-isogenic resistant and susceptible plants. In previous studies, the plant material had various origins including non-crop stands. Here, we compared plant growth and reproduction of the two types of plants in F2 and F3 segregating progenies that, on average, shared the same genetic background with the exception of the group of linkages in which the α2-tubulin gene is located. Indeed, the large number of F2 individuals used in the garden experiment or producing seeds for the growth cabinet and field experiments warranted similar and representative sampling of the genetic background in the two types of plants. The differences observed in the initial F2 could reflect the differences between the two parent species, thus indicating that the r allele could be linked with genes encoding for the tiller and secondary ear number. Similarly, there could be linkage between the S allele and genes encoding for the leaf width, ear length, and ear and grain weight. Indeed, tiller and secondary ears are typically more numerous in the green foxtail than in most of the foxtail millet cultivars, and reciprocally leaf width, ear length, and ear and grain weight are higher in the foxtail millet than in its wild relative (Darmency et al., 1987). The association between the r allele and short main ears/numerous secondary ears was completely lost in later generations after selection and crossing to a low tillering variety, which could indicate a simple linkage. In contrast, the association between the S allele and 1000-grain weight/grain weight of the main ear persisted through to the field experiment, which could either indicate a tight linkage or a pleiotropic effect of the gene.
The rr grains used in the growth cabinet, field experiment and those harvested in the field experiment were about 7–8% lighter than SS grains. This difference in grain weight could have caused the differential juvenile growth of F3 seedlings in the growth cabinet. However, even under high-density exacerbating competition, the rr plants were smaller and had fewer leaves than the SS plants, whereas plant weight still remained equivalent 38 DAS. This led us to believe that these differences were not a matter of resource reserves in the grains, but rather a difference in the growth kinetics. Moreover, the regression curves of plant height against leaf range revealed no difference between the two genotypes, thus indicating that grain weight had no impact on the initial seedling, at least at the earlier stage of the plant's life. These curves also show that the genetic growth program reached an equivalent stage in the two genotypes, therefore suggesting that the growth difference at 38 DAS was because of different plants’ biological clock driven by different cell division rates. This hypothesis is consistent with the role of α-tubulin in cell division processes (Baskin and Cande, 1990). Tubulin alteration in resistant Eleusine indica resulted in aberrations to some of the microtubule functions, including wall protuberances, incomplete cell division and a decrease in the overall number of microtubules (Vaughn, 1986). In Toxoplasma gondii, the spontaneous resistant mutant also showed increased rates of replication defects (Ma et al., 2008). Similar nuclear division defects were observed due to a β-tubulin mutation in Tetrahymena thermophila (Smith et al., 2004). However, microtubules are involved in many other processes, including cell wall expansion, intracellular transport and organelle positioning; alterations to any of these could also impact plant fitness.
The field experiment confirmed a slower leaf emission with lower stem elongation for the rr plants, both at the early stage and at maturity. The plant height difference was not observed in the initial interspecific F2 material, but it could have been masked by the wide variability generated in the F2 of the cross between small green foxtail and tall foxtail millet. Altered efficiency of the mutant α2-tubulin gene could cause such an effect on stem elongation. Similarly, the lower 1000-grain weight of the rr plants compared with the SS plants could indicate problems associated with cell division and grain replenishment. However, the lower 1000-grain weight alone cannot explain the lower main ear grain weight of the rr compared with the SS genotype. A reduced number of grains could also contribute to this result. Although grain number was lower in rr plants, the P-value was only marginally significant. Reduced main ear grain weight in rr plants could be because of a lower number of spikelets, but slower cell division kinetics in the rr genotype could hardly account for this effect. It could also arise from remains of the close linkage we previously reported between the α2-tubulin gene and a putative gametocide gene that resulted in loss of homozygous rr descendants (Tian et al., 2006). This possibility is consistent with a similar chromosome situation in rice, in which the corresponding OsTubA1 gene is located near two hybrid sterility and two gametocide genes. Alternately, some tubulin genes themselves are known to control pollen tube elongation (Yu et al., 2009), which could directly impact pollen success.
The yield penalty extrapolated from the difference in the main ear grain weight, which is generally the main source of grain production by low tillering cultivars, would be 20%. It is a fitness cost similar to that found for atrazine resistance in the same crop (Darmency and Pernès, 1989). We recently reported that trifluralin- and atrazine-resistant lines had similar yields in multisite experiments in China (Wang et al., 2010b), which is consistent with a comparable reproduction cost. In all, 20% is considered to be a significant yield penalty. However, a similar reduced yield in oilseed rape was not an obstacle to the use of atrazine-resistant cultivars, which have been cultivated on more than one million hectares in Australia for many years. The benefit of using an herbicide-resistant cultivar is good weed control, especially against weeds of the same botanical family. This results in less weed competition, which balances the potential low yield (Forcella, 1987). In the case of foxtail millet, the mutation also provides cross-resistance to some herbicides of the same mode-of-action, although the level of resistance is not high enough to preserve the crop from herbicide injury at herbicide dosages currently used for good weed control in the field (Wang and Darmency, 1997). Further research on compensatory mechanisms and tolerance gain from selection might include a search of the genetic resources of foxtail millet, which has been proposed as a possible tool to help overcome dinitroaniline-induced damages in soybean (Glover and Schapaugh, 2002). It would also be worthwhile to search the genetic resources of new mutants on both tubulin and non-tubulin genes, which actually occur spontaneously within a few generations in Toxoplasma gondii (Ma et al., 2008). Moderate yield reduction (5%) has been observed in genetically modified herbicide-resistant crops (Elmore et al., 2001) without hampering their widespread use. Many fitness costs linked to diverse traits in genetically modified crops have been reported, but they could be because of overexpression, silencing or breakage of native genes (Xia et al., 2010).
The consequence of such a high fitness cost in the absence of herbicide could be dramatic for mutant green foxtail. The difference of growth kinetics makes it less adapted to grow with the crop canopy, which results in receiving less light. The difference of seed weight makes it less suitable to develop seedlings in adverse conditions or results in smaller seedlings, thus possibly influencing the chance of reaching the adult stage and even reducing the adult reproduction (Moles and Westoby, 2004). The persistence of the trifluralin-resistant type in non-treated fields over 7 years (Andrews and Morrison, 1997) could be interpreted as the lack of S alleles in populations strongly submitted to the previous repeated treatments. As the resistance is recessive, the S alleles could not be transmitted through heterozygous plants and resulted cleared. In addition, green foxtail produces a huge quantity of seeds, and extremely dense infestations of resistant green foxtail were recorded up to 25 000 seedlings per m2 (Andrews and Morrison, 1997), which suggests that a large reserve of resistant seeds in the soil could reinfest the field for long time, even in the absence of the selection pressure. Such high exponential infestation dynamics was documented in triazine resistance spread in the fields (Darmency and Gasquez, 1990), and the resistant type is still found at high frequency in the infested fields 20 years after, in spite of the fitness cost (Gasquez, personal communication). In contrast, the fitness cost could explain the relatively low frequency of trifluralin resistance among weeds (Heap, 2010). Higher fitness costs were found in laboratory mutants showing herbicide resistance (Roux et al., 2004), but the most common situation is an absence of cost or undetectable costs (Vila-Aiub et al., 2009), and a case of higher seed production was even observed in Setaria (Wang et al., 2010a).
References
Andrews TS, Morrison IN (1997). The persistence of trifluralin resistance in green foxtail (Setaria viridis) populations. Weed Technol 11: 369–372.
Anthony RG, Hussey P (1999). Double mutation in Eleusine indica α-tubulin increase the resistance of transgenic maize calli to dinitroaniline and phosphorothioamidate herbicides. Plant J 18: 669–674.
Anthony RG, Reichelt S, Hussey P (1999). Dinitroaniline herbicide-resistant transgenic tobacco plants generated by co-overexpression of a mutant α-tubulin and a β-tubulin. Nature Biotech 393: 260–263.
Ashton FM, Crafts AS (1981). Dinitroanilines. In: FM Ashton and AS Crafts (eds) Mode of Action of Herbicides. John Wiley & Sons, New York, 201–223.
Baskin TI, Cande WZ (1990). The structure and function of the mitotic spindle in flowering plants. Annu Rev Plant Physiol Plant Mol Biol 41: 277–315.
Beversdorf WD, Hume DJ, Donnelly-Vanderloo MJ (1988). Agronomic performance of triazine-resistant and susceptible reciprocal spring canola hybrids. Crop Sci 28: 932–934.
Boerboom CM, Lauer JG (1997). Performance of imazethapyr-resistant corn (Zea mays) compared with susceptible near-isogenic commercial hybrids. Weed Tech 11: 110–117.
Caretto S, Giadina MC, Nicolodi C, Mariotti D (1994). Chlorsulfuron resistance in Daucus carota cell lines and plants: involvement of gene amplification. Theor Appl Genet 88: 520–524.
Darmency H, Gasquez J (1990). Appearance and spread of triazine resistance in common lambsquarters (Chenopodium album). Weed Technol 4: 173–177.
Darmency H, Pernès J (1989). Agronomic performance of triazine resistant foxtail millet (Setaria italica (L.) Beauv.). Weed Res 29: 147–150.
Darmency H, Zangre GR, Pernès J (1987). The wild-weed-crop complex in Setaria: a hybridization study. Genetica 75: 103–107.
Délye C, Menchari Y, Michel S, Darmency H (2004). Molecular bases for sensitivity to tubulin-binding herbicides in green foxtail. Plant Physiol 136: 3920–3932.
Duke SO (1996). Herbicide-resistant Crops. CRC Press, Boca Raton.
Elmore RW, Roeth FW, Nelson LA, Shapiro CA, Klein RN, Knezevic SZ et al. (2001). Glyphosate-resistant soybean cultivar yields compared with sister lines. Agron J 93: 408–412.
Forcella F (1987). Herbicide-resistant crops: yield penalties and weed thresholds for oilseed rape (Brassica napus L.). Weed Res 27: 31–34.
Glover DG, Schapaugh Jr WT (2002). Inheritance of resistance to pendimethalin herbicide induced stem damage in soybean. Euphytica 125: 433–437.
Harris JR, Gosset BJ, Toler JE (1995). Growth characteristics of selected dinitroaniline-resistant and -susceptible goosegrass (Eleusine indica) populations. Weed Technol 9: 561–567.
Heap IM (2010). The international survey of herbicide resistant weeds. Online Internetwww.weedscience.com (accessed April).
Jasieniuk M, Brûlé-Babel A, Morrison IN (1994). Inheritance of trifluralin resistance in green foxtail (Setaria viridis). Weed Sci 42: 123–127.
Landi P, Frascaroli E, Guilani MM (1999). Genetic variability for resistance to trifluralin in Zea mays. Weed Sci 47: 369–374.
Lowe DB, Swire-Clark GA, McCarty LB, Whitwell T, Baird WV (2001). Biology and molecular analysis of dinitroaniline-resistant Poa annua L. Int Turfgrass Soc Res 9: 1019–1025.
Ma C, Li C, Ganesan L, Oak J, Tsai S, Sept D et al. (2007). Mutations in α-tubulin confer dinitroaniline resistance at a cost to microtubule function. Mol Biol Cell 18: 4711–4720.
Ma C, Tran J, Li C, Ganesan L, Wood D, Morrissette N (2008). Secondary mutations correct fitness defects in Toxoplasma gondii with dinitroaniline resistance mutations. Genetics 180: 845–856.
Massinga RA, Al-Khatib K, St. Amand P, Miller JF (2005). Relative fitness of imazamox-resistant common sunflower and prairie sunflower. Weed Sci 53: 166–174.
Moles AT, Westoby M (2004). Seedling survival and seed size: a synthesis of the literature. J Ecol 92: 372–383.
Morrison IN, Todd BG, Nawolsky KM (1989). Confirmation of trifluralin-resistant green foxtail (Setaria viridis) in Manitoba. Weed Technol 3: 544–551.
Murphy TR, Gossett BJ, Toler JE (1986). Growth and development of dinitroaniline-susceptible and -resistant goosegrass (Eleusine indica) biotypes under noncompetitive conditions. Weed Sci 34: 704–710.
Purrington CB, Bergelson J (1997). Fitness consequences of genetically engineered herbicide and antibiotic resistance in Arabidopsis thaliana. Genetics 145: 807–814.
Roux J, Gasquez J, Reboud X (2004). The dominance of the herbicide resistance cost in several Arabidopsis thaliana mutant lines. Genetics 166: 449–460.
Smith JJ, Yakisich JS, Kapler GM, Cole ES, Romero DP (2004). A β-tubulin mutation selectively uncouples nuclear division and cytokinesis in Tetrahymena thermophila. Eukaryotic Cell 3: 1217–1226.
Tan S, Evans RR, Dahmer ML, Singh BK, Shaner DL (2005). Imidazolinone-tolerant crops: history, current status and future. Pest Manag Sci 61: 246–257.
Tian X, Délye C, Darmency H (2006). Molecular evidence of biased inheritance of trifluralin herbicide resistance in foxtail millet. Pl Breed 125: 254–258.
Valverde BE, Radosevich SR, Appleby AP (1988). Growth and competitive ability of dinitroaniline-herbicide resistant and susceptible goosegrass (Eleusine indica). Proc West Soc Weed Sci 41: 81.
Vaughn KC (1986). Cytological studies of dinitroaniline-resistant Eleusine. Pest Biochem Physiol 26: 66–74.
Vila-Aiub MM, Neve P, Powles SB (2009). Fitness cost associated with evolved herbicide resistance alleles in plants. New Phytol 184: 751–767.
Wang T, Darmency H (1997). Dinitroaniline herbicide cross-resistance in resistant Setaria italica lines selected from interspecific cross with S. viridis. Pest Sci 49: 277–283.
Wang T, Fleury A, Ma J, Darmency H (1996). Genetic control of dinitroaniline resistance in Foxtail Millet (Setaria italica). J Hered 87: 423–426.
Wang T, Picard JC, Tian X, Darmency H (2010a). A herbicide-resistant ACCase 1781 Setaria mutant shows higher fitness than wild type. Heredity 105: 394–400.
Wang T, Shi Y, Li Y, Song Y, Darmency H (2010b). Population growth rate of Setaria viridis in absence of herbicide: resulting yield loss in S. italica, the foxtail millet. Weed Res 50: 228–234.
Xia H, Chen Liangyan, Wang F, Lu BR (2010). Yield benefit and underlying cost of insect-resistance transgenic rice: implication in breeding and deploying transgenic crops. Field Crops Res 118: 215–220.
Yemets AI, Blume YB (2007). Mutant genes of plant tubulins as selective marker genes for genetic engineering. Cytol Genet 41: 156–166.
Yu YL, Li YZ, Li LL, Lin JX, Zheng C, Zhang LY (2009). Overexpression of PwTUA1, a pollen-specific tubulin gene, increases pollen tube elongation by altering the distribution of α-tubulin and promoting vesicle transport. J Exp Bot 60: 2737–2749.
Acknowledgements
The authors are especially grateful to A Fleury for field experiments and seed stock management. Research grants were provided by the Burgundy Region Council and researcher exchanges by the French-Chinese Association for Scientific Research (AFCRST) PRA contract.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Darmency, H., Picard, J. & Wang, T. Fitness costs linked to dinitroaniline resistance mutation in Setaria. Heredity 107, 80–86 (2011). https://doi.org/10.1038/hdy.2010.169
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hdy.2010.169