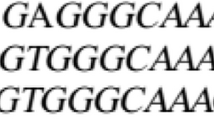Abstract
It is often alleged that mutations conferring herbicide resistance have a negative impact on plant fitness. A mutant ACCase1781 allele endowing resistance to the sethoxydim herbicide was introgressed from a resistant green foxtail (Setaria viridis (L.) Beauv) population into foxtail millet (S. italica (L.) Beauv.). (1) Better and earlier growth of resistant plants was observed in a greenhouse cabinet. (2) Resistant plants of the advanced BC7 backcross generation showed more vigorous juvenile growth in the field, earlier flowering, more tillers and higher numbers of grains than susceptible plants did, especially when both genotypes were grown in mixture, but their seeds were lighter than susceptible seeds. (3) Field populations originating from segregating hybrids had the expected allele frequencies under normal growth conditions, but showed a genotype shift toward an excess of homozygous resistant plants within 3 years in stressful conditions. Lower seed size, lower germination rate and perhaps unexplored differences in seed longevity and predation could explain how the resistant plants have the same field fitness over the whole life cycle as the susceptible ones although they produce more seeds. More rapid growth kinetics probably accounted for higher fitness of the resistant plants in adverse conditions. The likelihood of a linkage with a beneficial gene is discussed versus the hypothesis of a pleiotropic effect of the ACCase resistance allele. It is suggested that autogamous species like Setaria could not develop a resistant population without the help of a linkage with a gene producing a higher fitness.
Similar content being viewed by others
Introduction
Mutations conferring resistance to xenobiotics are generally expected to incur a fitness cost in the absence of this selection pressure (Coustau et al., 2000; Andersson, 2006). The question of ‘fitness cost’ has long been discussed for wild populations of any organism acquiring resistance genes to antibiotics, pesticides and pests and is still a hot debate involving important evolutionary and applied perspectives (Andersson, 2006; Ffrench-Constant, 2007; Orgil et al., 2007). Fitness can be defined as the ability to reproduce to the next generation. In the case of herbicide resistance, fitness costs in the absence of the relevant herbicide can help explain the low frequency of resistance alleles and may guide field management to control resistant weeds (Gressel, 2002; Menchari et al., 2008; Vila-Aiub et al., 2009). Theoretical developments have been tested using laboratory-mutated plant material in greenhouse conditions, giving insights into the role of fitness and the best methods of measurement (Roux et al., 2004, 2006). In the field, spontaneously occurring herbicide-resistant mutants showed fitness costs ranging from not detectable or negligible (Tranel and Wright, 2002; Délye, 2005) to around 19–25% (Beversdorf et al., 1988; Darmency and Pernès, 1989) or even greater (Tardif et al., 2006). It is important to note that for herbicide resistance increased fitness of resistant individuals has never been reported.
In the case of resistance to acetyl coenzyme A carboxylase (ACCase)-inhibitors, the most widespread mutation, ACCase 1781, showed no or negligible fitness cost in Lolium rigidum and Alopecurus myosuroides (Vila-Aiub et al., 2005b; Menchari et al., 2008). These two species have developed numerous resistant populations in Australia and Europe, respectively, which fits well with the lack of noticeable fitness cost (Délye, 2005). The same ACCase 1781 mutation also occurred in Setaria viridis (Délye et al., 2002), the green foxtail, a widespread self-pollinated weed. Surprisingly, a preliminary field study showed that resistant backcrossed plants (BC2) of S. viridis to S. italica, the foxtail millet, its domesticated form, showed higher seed production than their susceptible counterpart (Wang and Darmency, 1996). The aim of this paper is to investigate with more appropriate plant material the nature and consequences of this singularity. We report on the impact of the ACCase 1781 mutation (conferring resistance to ACCase inhibitors) on the overall fitness of advanced backcross generations of Setaria and of segregating populations issuing from S. italica × S. viridis crosses.
Differential fitness between herbicide-resistant and susceptible plants must be measured under competitive situations in field conditions, using isogenic materials and over the whole life cycle (Gressel, 2002; Vila-Aiub et al., 2009). First, the importance of the growth conditions was illustrated by Purrington and Bergelson (1997) who showed a significant 31% reduction of seed production for a Pro-197-Ser Arabidopsis thaliana mutant resistant to ALS inhibitors in non-fertilized field conditions whereas no difference was found when the fertilizer was used. Here, we measured fitness components in a growth cabinet and in the field with two plant densities and pure vs mixed planting of resistant and susceptible BC7 to provide various levels of competition among plants. Second, isogenic lines have been demonstrated to be of prime importance for clear-cut results. In particular, backcrossing to a closely related crop has already been successfully used in other studies (Beversdorf et al., 1988; Darmency and Pernès, 1989; Purrington and Bergelson, 1997). This also has practical advantages such as ease of backcrossing by using a male sterile line of the crop, and lack of seed dormancy to reduce heterogeneity in seedling emergence and growth rates. The study of segregating F2 populations (Menchari et al., 2008) is another option. We used both types of material in our experiments. Finally, estimation of fitness differences over a whole life cycle or even several generations has also been recommended as a definitive measure to quantify gene effects (Plowman et al., 1999, Roux et al., 2006). To do this, we carried out a 3-year field experiment to detect gene frequency changes in progeny populations of the interspecific hybrids in stressful and control field conditions.
Materials and methods
Plant material
Plants from a population of Setaria viridis (L.) Beauv that had evolved high resistance to an ACCase inhibitor herbicide (sethoxydim) in Canada (UM131, Heap and Morrison, 1996) were crossed to S. italica (L.) Beauv. (Wang and Darmency, 1997). The sethoxydim resistance was demonstrated to be dominant (Wang and Darmency, 1997) and because of a point mutation in the carboxyltransferase domain of the nuclear gene encoding a plastidic ACCase isoform causing an isoleucine–leucine residue substitution at position 1781 (Délye et al., 2002). Fertile hybrids (ms/ms) between a female germplasm, line Shda-1 heterozygous for a dominant male sterility (MS/ms) and sethoxydim-resistant green foxtail (ms/ms) were used as pollen donors, backcrossing to female heterozygous MS/ms Shda-1 plants to produce a BC1 line (Figure 1). MS BC1 individuals were discarded upon examination of the anthers. About 300 male fertile (ms/ms) BC1 were used to produce BC2 offspring with 300 heterozygous MS/ms Shda-1, enclosing only one ear of each parent in the same bag and then harvesting the ears separately. Resistant BC1 were identified after harvest as their seeds segregated into resistant (R) and susceptible (S, see the section RR, RS and SS identification), and then the corresponding Shda-1 ears were selected as retaining both susceptible and resistant BC2 seeds. The same procedure, always with 300 pairs of plants, was used to produce BC3, and repeated again up to the BC7 generation. Fertile R BC7 were self-fertilized to get F2 BC7. Finally, 150 homozygous RR and 150 SS F2 BC7 were self-fertilized to obtain pure RR and SS F3 BC7 seed lots (Figure 1). The seed weight of SS F3 BC7 was higher than that of RR F3 BC7: 3.28±0.04 g (s.e.m.) versus 3.06±0.03 g for 1000 seeds. These seeds were used for the growth cabinet and the field experiments.
Breeding scheme to produce the F3BC7 materials used in the field experiment (in the boxes). Genotypes appearing in the progeny but not used in the breeding plan are not indicated. R and S are the herbicide-resistant dominant and susceptible recessive alleles, respectively. MS and ms are the dominant male sterility and recessive male fertile alleles, respectively.
For the populations experiment, a homozygous fertile, resistant fifth-generation germplasm was used as a pollen donor to pollinate a susceptible green foxtail (from Burgundy, France) and to obtain F1 hybrids. F2 and F3 were subsequently obtained by self-pollination of 5 F1 and 38 F2, respectively.
RR, RS and SS identification
Total DNA extraction of a leaf segment allowed an allele-specific PCR test to identify homozygous susceptible (SS) and resistant (RR) and heterozygous-resistant (RS) genotypes, as described by Délye et al., (2002). Before this technique was made available, the genotype was identified following harvest by characterization of the progeny. Fifty seeds of each ear were germinated in a Petri dish on a filter paper moistened with commercial sethoxydim solution at 90 μM sethoxydim (Fervinal, Schering/Agro-Végétal, France). All the seeds of a susceptible plant (SS) germinated but died without the seminal roots elongating, whereas for homozygous resistant plants (RR) all the seeds developed normal roots. A mixture of about 75/25% normal/dead seedlings was observed for heterozygous-resistant plants (RS).
Growth cabinet experiment
A preliminary experiment was designed to test the seed germination on moist paper in 11 cm large Petri dishes at 27 °C in the dark. Twenty-five seeds of each RR and SS F3 BC7 genotype were placed in each 14 cm-diameter Petri dish (16 replicates), and the length of the radicle and the primary shoot was measured 48 h after sowing. For the growth experiment, the RR and SS F3 BC7 seeds were separately sown in 8-cm square pots filled with 200 g of a sandy clay soil. After 7 days in a growth cabinet at 27 °C during a 16 h day (200 μM−2 s−1) and 22 °C during the night, the emerged seedlings were thinned to 1 or 3 per pot, creating two levels of plant density to provide different levels of competition. The pots were grouped by density and placed in three growth cabinets, each one containing the two groups, and each group containing both R and S plants. The pots were given 22 ml of water and their positions were randomly changed within a density group every other day. This experiment mimics what could occur in a mixed population at densities between 150 and 450 plants m−2, at similar spacing but 8–9 times higher densities than in the field experiment (see the section field experiment with BC7). The plant height at the last visible leaf/stem angle, the number of visible leaves and the dry aboveground biomass were measured 38 days after planting. The data were analyzed with a fixed-effects model ANOVA for a split-plot design including three blocks (growth cabinets), two densities (main plots) and two genotypes.
Field experiment with BC7
F3 BC7 seeds from the same batch as those used for the growth cabinet experiment were directly sown in a randomized split-plot arrangement with two densities, 20 and 50 plants m−2, which corresponded to densities of foxtail millet production in France and China with 0.75 and 0.37 m inter-row, respectively, and five replicate blocks. Within main plots for each density, there were pure RR and SS and mixed RR/SS subplots. Each subplot consisted of three rows 5 m long, from the central row of which the six central plants were measured (or 6 RR and 6 SS for mixed planting) to avoid any border effect. For mixed planting, pockets of five seeds of the RR and SS genotype were alternately sown along each row every 7 or 5 cm according to the density. Thinning occurred to result in only one seedling at each place. For the pure plantings, 300 seeds were planted per row, and emerged seedlings were counted before being thinned to the desired density. The plants were measured for height to the last leaf node and leaf and tiller numbers, at two dates (48 days after emergence and at maturity) to detect any differential growth kinetics. The date of flowering was recorded as when an ear emerged from the main stem. Flag-leaf length and width, and aboveground dry biomass (excluding ears) were measured at maturity. The reproductive traits measured were the total grain number and grain weight per plant. We also included measurements of the length of the main ear, the number of fertile tillers, and the weight of 1000 grains to describe components of reproductive allocation. Average data of the six plants measured per treatment and replicate were subjected to a fixed-effects model ANOVA for a split-plot design comprising blocks, densities (D) as main plots, and genotypes (G) and planting (P) factors as subplots, with G × D, G × P, D × P and G × D × P interactions, using SYSTAT software version 10 (SPSS Inc. 1999). Levene's test for heterogeneity of variances was never significant, thus allowing the use of untransformed data.
Populations experiment
F2 seeds were planted in 2002 in the field at a density of 25 seeds m−2 on five 3 × 3 m plots, and F3 seeds at 240 seeds m−2 on five plots. Before planting, three of the plots for F2 and the three plots for F3 were sprayed with half the field dose of trifluralin (400 g a.i. ha–1, Treflan EC, Dow AgroScience, France) to create a stressful environment at emergence time because trifluralin is a cell division inhibitor used as a pre-emergence soil-incorporated herbicide. Two plots for each F2 and F3 were kept free of herbicide as control. The soil surface was gently disturbed with a rake just after spraying and planting occurred immediately. The plots were surrounded by two rows of maize to prevent pollen and seed migration. The experiment was kept free of other weeds by hand weeding without disturbing the test plants. The plants produced seeds naturally and no harvest or soil movement practices were performed. The plots were maintained in 2003 and 2004 without intervention, except for the planting of border maize plants and application of the trifluralin half-dose treatment. A leaf segment of a sample of the plants growing in the plots was collected in July 2004 to run routine allele-specific PCR tests to identify the SS, RR and RS genotypes as described by Délye et al., (2002) for ACCase inhibitors resistance. Seeds of the initial F2 and F3 populations were sown in Petri dishes, and the first leaf of the seedlings, or the seed if not germinated, was used to determine their genotype by PCR.
Results
Growth cabinet experiment
Germination in Petri dishes was 98% for the two genotypes. Despite the fact that the R seeds weighed less than the S seeds, at 2 days after sowing, the radicle of R seeds was significantly larger than that of the S seeds (2.67±0.04 versus 2.42±0.04 cm, t=4.7, P<0.001). No significant difference was evident for the primary shoot (t=0.66, P=0.50). Germination in pots was 95.7 and 87.3% for S and R seeds, respectively. By 38 days after planting, R plants were taller and heavier than S plants regardless of the density (Table 1). This confirmed the previous observation where the R seedlings of the BC2 lines had more vigorous growth than the S seedlings (Wang and Darmency, 1996). However, there was no difference in the number of leaves between R and S plants, which indicated no phyllochrone difference, but rather different resource-use efficiency. The density effect was significant, indicating that plants at this juvenile period were sensitive to competition.
Field experiment with BC7
Seedling emergence was higher for S seedlings than for R BC7: 78.6±2.2 versus 70.9±3.8%. For both genotypes, most of the characters measured at maturity, except the plant height and the 1000-grain weight showed significantly lower values at the higher plant density than at the lower plant density (Figure 2). The average grain yield per plant was two times higher at 20 plants m−2, (34.7 g) than at 50 plants m−2 (16.6 g), but this resulted in higher yield per m2 at 50 plants m−2 (0.83 versus 0.69 kg m−2). The planting type affected five of the 15 characters, but in only two instances were these effects independent of the genotype; the length and width of the flag leaf were larger in mixed planting than in pure stand, thus showing a typical morphology response to inter-biotype competition (Table 2). The genotype effect was significant for 10 of the 15 characters, and most of them showed interactions with the density or the planting type. The R plants produced consistently 24% more grains than the S, whatever the density and planting type (Table 2).
Main components of fitness of the R (filled bars) and S (open bars) F3BC7 materials in mixed stands at two plant densities in the field experiment. (a) percentage of seedling emergence. (b) number of tillers at maturity. (c) number of grains per plant. (d) weight of 1000 grains (g). Error bars indicate standard error of the mean (n=5).
The early records at 48 days after emergence showed no difference between the two genotypes in pure planting. When grown in mixture, the R plants were taller and had more leaves and tillers, they flowered earlier, had fewer leaves at flowering, and they were shorter at maturity than the S plants (Table 2). These results agree with the data from the growth cabinet experiment (Table 1). This indicates more competitive juvenile growth of the R plants than of the S plants, with earlier maturity in mixed planting than occurred in the pure stand. In both planting types, the R plants had smaller leaves than S plants, but the final total grain weight and dry biomass of R and S plants were not different. The R plants had more tillers but they produced lighter grains than S plants in mixed planting, whereas again no difference was found in pure planting (Figure 2). The reproductive efficiency of the R plants in terms of dry biomass was higher than that of the S plants, especially in mixed planting (Table 2). Finally, an interaction between the genotype and density factors was observed in two instances (Table 2). In particular the number of grains released per dry biomass unit was similar between R and S plants at the density of 50 plants m−2, (260 and 244, respectively), but significantly different at the 20 plants m−2 density (377 and 288, respectively).
Populations experiment
The genotype ratio of the sowed F2 population used to establish the populations experiment was not different from the expected 1:2:1 hypothesis (Table 3). After 2 years, results among plots were homogeneous among the two F4 controls on the one hand, and the three stressed plots on the other hand (χ2=1.45 and 6.23 non-significant at P<0.05, respectively). The frequencies in control and stressed plots of F4 were different (χ2=29.1, P<0.001), with more RR and fewer SS in the stressed plots than in the controls. Taking the genotype frequencies of the sown F2 as the starting points of the further F4 experimental populations, we calculated the expected genotype ratios of the F4 generation 2 years later. For that purpose, we assumed that (1) all the seeds germinated without dormancy (that is, no generation overlap); (2) all the genotypes had the same seed productivity; (3) segregation of heterozygotes was unbiased; and (4) the plants were completely self-pollinated. The results observed in the control plots showed no difference from the expected ratios (Table 3). In contrast, a large excess of RR and a lack of SS were observed in the stressed plots.
Similar results were obtained with the F5 populations derived from the F3, except that, unfortunately, the control plots were accidentally destroyed. In addition, the sowed F3 seeds obtained from 38 random F2 plants already showed a deficit of the homozygous SS genotype when compared with the F2 seed composition. After 2 years, the results among the three plots were homogeneous (χ2=3.99 non-significant at P<0.05). Taking the F3 genotype frequencies as a reference point, the stressed F5 populations were richer than expected in the RR and lacking in the SS genotype (Table 3).
Discussion
Earlier studies on the fitness cost of resistance to ACCase inhibitor herbicides in a species closely related to foxtail millet, Setaria faberi, showed no differences in the competitive abilities of the resistant versus susceptible accessions (Wiederholt and Stoltenberg, 1996). A similar result was found in Lolium rigidum populations (Gill et al., 1996), but these two studies did not control the genetic background, and the ACCase resistance mechanism of the resistant plant material was unknown and could therefore have been any of the many mechanisms that endow ACCase resistance. Studies with ACCase herbicide-resistant Lolium rigidum also showed no significant difference between plants with the ACCase 1781 mutation versus plants without this mutation (Vila-Aiub et al., 2005a), apart from results for germination (Vila-Aiub et al., 2005b). Different mutants of the same ACCase gene in a homogeneous genetic background have been shown to display different fitness costs in Alopecurus myosuroides, but again the ACCase-1781 mutant showed no fitness cost (Menchari et al., 2008). These results show the critical importance of accurate knowledge of the exact ACCase gene mutation, the use of isogenic R and S lines and the use of a range of measurements over the whole plant life cycle to be able to detect for any small differences in fitness.
Our earlier work to compare R versus S plants involved repeated backcrossing to the crop parent, and we showed with BC2 plants an apparent superiority of heterozygous (RS) plants as regards earlier flowering and the number of grains (Wang and Darmency, 1996). The same results were obtained here with nearly isogenic homozygous R and S descendants of the BC7 generation. The BC7 generation produced R seeds that were on average 10% lighter than S seeds. However, notwithstanding this difference, 38 days after sowing, the R seedlings grew faster than S seedlings in the growth cabinet experiment. The field experiment confirmed superior growth of the R plants at the early growth stage. The R plants showed higher growth kinetics, matured earlier and they flowered earlier and eventually produced more tillers and 24% more seeds than the S plants. This situation is summarized in Figure 2 showing the main fitness components under mixed planting. There was a trade-off between tiller and seed production on the one hand and seed weight and seedling emergence on the other hand. This is the first study with herbicide-resistant plants, which shows that herbicide-resistant R plants can display superior fitness components than their S counterparts.
From an ecological perspective, these differences could have important consequences for the weed green foxtail. Lower seed size could cause lower seed survival and lower emergence rate (Moles and Westoby, 2004). Although the backcrossed isogenic material studied here was not typically weedy, lower seed size caused lower emergence rates in field evaluation, which may reveal the behaviour of the wild R green foxtail parent. In the second approach reported here, the populations experiment, the R and S plants were not isogenic, but shared the same genetic background, on average, as they belonged to the same segregating F2 and F3 populations. The progeny of the F2 population 2 years after seeding, in a herbicide-free environment (without trifluralin), had the expected genotype frequencies under the assumption of no differential fitness. Therefore, it is likely that the lower emergence potential that we observed for the R plants, allied to a lower seed survival, could have counterbalanced their higher seed production demonstrated in the experiments using the BC7s. Differences of dynamics of seed germination and seedling emergence have already been proposed to affect the fitness of the herbicide-resistant 1781-ACCase mutant of Lolium rigidum. In the study of the identified 1781-ACCase mutation, the R biotype had slower germination than S plants (Vila-Aiub et al., 2005b). Variable results were observed in two other studies, but using non-isogenic materials and without knowledge of the ACCase resistance mechanism (Gill et al., 1996; Recasens et al., 2007). In addition, traits such as seed viability and longevity that were not measured in our experiment but are pertinent to the whole life cycle, have the potential to account for the observed lack of fitness difference between the S and R plants under field plots without trifluralin stress. Huge seed losses occur in Setaria and this can have a critical impact on demography (Wang et al., 2010). For instance, the earlier flowering and seed set of R plants could expose R seeds to longer periods of predation than S seeds.
In our study, the increase in frequencies of RR plants relative to SS plants in the F4- and F5-stressed field populations clearly showed a fitness benefit for the R allele. The dose of trifluralin was low enough for it not to be a direct mortality factor, but certainly acted at emergence time to select for the most vigorous seedlings because trifluralin is a cell division inhibitor. Resistance to sethoxydim is not known to confer any differential response to trifluralin, for which an independent, unlinked resistance gene is known (Shi et al., 2008). A larger radicle at germination and higher juvenile growth of the R seedlings, as observed in the growth cabinet experiment, probably conferred some advantage for escaping the trifluralin-contaminated soil layer. Other stressful conditions such as high plant density and mixed stands increased the relative fitness of the R plants versus the S plants in our field experiments. The emergence and early seedling stages, again, were pinpointed here, which could be due to a differential effect of R and S ACCase alleles through, for instance, differential early availability of fatty acid chains. However, this is inconsistent with the finding that in vitro activities of the ACCase of R (the same UM131 accession) and S plants are not different (Shukla et al., 1997). Yu et al., (2007) provided further evidence that the 1781 mutation does not impair ACCase function in Lolium rigidum.
The ACCase gene encoding for the herbicide resistance has well known important functions in fatty acid synthesis (Délye, 2005), so that some direct pleiotropic effects on growth could be expected if the mutation conferring resistance imparts some functional change. However, the details showed that the R and S lines differed significantly in 12 out of 15 characters (including the differences observed in interaction with density and planting effects), and the link between these 12 differences and the R allele is unlikely. It is therefore possible that genes encoding these differences and the ACCase gene are very closely linked and inherited together, even though seven generations of backcrossing involving a large number of progenitors provided considerable opportunity for crossing-over. In theory, and given the high number of progenitors used at each generation, at the seventh backcrossed generation, the BC7 should retain no more than 0.4% of the wild parent genome. Higher numbers of tillers and smaller-sized grains could represent persistence of the wild parent characters, which have possibly been conserved during the backcrossing process. It is also possible that all the differences may have been the consequence of differential plant growth at earlier stages. For instance, the genotype × planting interaction, together with a faster development of R seedlings within the first 48 days, showed that mixed R and S plants began competing early and R plants grew better than S plants. This early growth vigour, associated with early flowering, could had consequences such as lower height at maturity, higher tillering, higher total number of grains, and finally a lower resource allocation per grain resulting in lighter grains for R plants than for S plants. These differences could have been more or less exacerbated according to the plant density, as observed in other case studies (Pedersen et al., 2007) or suggested by the genotype × planting interaction in the BC7 experiment.
To our knowledge, higher fitness for resistant plants has never before been reported in the literature, even in studies for which the resistance mechanism is unknown (Gill et al., 1996; Wiederholt and Stoltenberg, 1996; Vila-Aiub et al., 2005b, ; Recasens et al., 2007; Menchari et al., 2008). This strongly suggests a gene linkage in our plant material. In the case of Setaria, perhaps only one other case of the ACCase 1781 mutant has been reported, as identified by cross-resistance patterns to ACCase inhibitors (Shukla et al., 1997; Beckie et al., 1999). In contrast, it is noteworthy that numerous ACCase 1781 mutant populations were found for Lolium and Alopecurus (Heap, 2009), and even in several different ACCase haplotypes in Alopecurus (Délye et al., 2004). If the ACCase 1781 mutant actually confers some fitness cost under stressed environments, it is likely that high allogamy in Lolium and Alopecurus would allow a rapid match of the R mutant with genotypes compensating for this fitness cost during the seed phase of the life cycle, thus enabling more populations to display this resistance. In this case the apparent lack of fitness cost of the ACCase 1781 mutants in Lolium and Alopecurus would be the result of an evolutionary compensatory process. In contrast, a linkage of the mutant ACCase with a beneficial gene could provide a rare situation where the fitness cost of herbicide resistance is masked under stressed field conditions, which allows the development of a resistant population of the self-pollinated Setaria species. To support the co-segregating genes hypothesis as the major factor leading to the occurrence of resistant Setaria mutants, it would be necessary to study more resistant populations and show evidence of similar linkage between the mutant ACCase 1781 allele and a beneficial gene. However, few such populations are available. A more feasible approach could be the introduction of the 1781 allele into a susceptible background via genetic transformation to study the effect of the mutated gene by itself, as in Purrington and Bergelson (1997). Finally, research on similar co-segregating genes in another autogamous species that has evolved an ACCase 1781 mutant, such as Avena fatua (Christoffers et al., 2002; Heap, 2009), could confirm the importance of this mating system for the appearance and spread of herbicide resistance.
References
Andersson DI (2006). The biological cost of mutational antibiotic resistance: any practical conclusions? Curr O Microbiol 9: 461–466.
Beckie HJ, Thomas AG, Légère A (1999). Nature, occurrence, and cost of herbicide-resistant green foxtail (Setaria viridis) across Saskatchewan ecoregions. Weed Technol 13: 626–631.
Beversdorf WD, Hume DJ, Donnelly-Vanderloo MJ (1988). Agronomic performance of triazine-resistant and susceptible reciprocal spring canola hybrids. Crop Sci 28: 932–934.
Christoffers M, Berg ML, Messersmith CG (2002). An isoleucine to leucine mutation in acetyl-CoA carboxylase confers herbicide resistance in wild oat. Genome 45: 1049–1056.
Coustau C, Chevillon C, Ffrench-Constant R (2000). Resistance to xenobiotics and parasites: can we count the cost ? Trends Ecol Evol 15: 378–383.
Darmency H, Pernès J (1989). Agronomic performance of triazine resistant foxtail millet (Setaria italica (L Beauv.)). Weed Res 29: 147–150.
Délye C, Wang T, Darmency H (2002). An isoleucine-leucine substitution in chloroplastic acetyl-CoA carboxylase from green foxtail (Setaria viridis L Beauv.) is responsible for resistance to the cyclohexanedione herbicide sethoxydim. Planta 214: 421–427.
Délye C (2005). Weed resistance to acetyl coenzyme A carboxylase inhibitors: an update. Weed Sci 53: 728–746.
Délye C, Straub C, Michel S, Le Corre V (2004). Nucleotide variability at the acetyl coenzyme A carboxylase gene and the signature of herbicide selection in the grass weed Alopecurus myosuroides (Huds.). Mol Biol Evol 21: 884–892.
Ffrench-Constant RH (2007). Which came first: insecticides or resistance? Trends in Genetics 23: 1–4.
Gill GJ, Cousens RD, Allan MR (1996). Germination, growth and development of herbicide resistant and susceptible populations of rigid ryegrass (Lolium rigidum). Weed Sci 44: 252–256.
Gressel J (2002). Molecular Biology of Weed Control. Taylor & Francis: London.
Heap IM, Morrison IN (1996). Resistance to aryloxyphenoxypropionate and cyclohexanedione herbicides in green foxtail (Setaria viridis). Weed Sci 44: 25–30.
Heap IM (2009). The international survey of herbicide-resistant weeds. Online Internet www.weedscience.com (accessed September).
Menchari Y, Chauvel B, Darmency H, Délye C (2008). Fitness cost associated with three mutant acetyl-coenzyme A carboxylase alleles endowing herbicide resistance in black-grass Alopecurus myosuroides. J Appl Ecol 45: 939–947.
Moles AT, Westoby M (2004). Seedling survival and seed size: a synthesis of the literature. J Ecol 92: 372–383.
Orgil U, Araki H, Tangchaiburana S, Berkey R, Xiao SY (2007). Intraspecific genetic variations, fitness cost and benefit of RPW8, a disease resistance locus in Arabidopsis thaliana. Genetics 176: 2317–2333.
Pedersen BP, Neve P, Andreasen C, Powles SB (2007). Ecological fitness of a glyphosate-resistant Lolium rigidum population: growth and seed production along a competition gradient. Basic Appl Ecol 8: 258–268.
Plowman AB, Richards A.J, Tremayne MA (1999). Environmental effects on the fitness of triazine-resistant and triazine-susceptible Brassica rapa and Chenopodium album in the absence of herbicide. New Phytol 141: 471–485.
Purrington CB, Bergelson J (1997). Fitness consequences of genetically engineered herbicide and antibiotic resistance in Arabidopsis thaliana. Genetics 145: 807–814.
Recasens J, Caimons O, Torra J, Taberner A (2007). Variation in seed germination and early growth between and within Co A Carboxylase herbicide resistant and susceptible Lolium rigidum accessions. Seed Sci Technol 35: 32–47.
Roux J, Gasquez J, Reboud X (2004). The dominance of the herbicide resistance cost in several Arabidopsis thaliana mutant lines. Genetics 166: 460–499.
Roux F, Giancola S, Durand S, Reboud X (2006). Building of an experimental cline with Arabidopsis thaliana to estimate herbicide fitness cost. Genetics 173: 1023–1031.
Shi Y, Wang T, Li Y, Darmency H (2008). Impact of transgene inheritance on the mitigation of gene flow between crops and their wild relatives: the example of foxtail millet. Genetics 180: 969–975.
Shukla A, Leach GE, Devine MD (1997). High-level resistance to sethoxydim conferred by an alteration in the target enzyme, acetyl-CoA carboxylase, in Setaria faberii and Setaria viridis. Plant Physiol Biochem 35: 803–807.
Tardif FJ, Rajcan I, Costea M (2006). A mutation in the herbicide target site acetohydroxyacid synthase produces morphological and structural alterations and reduces fitness in Amaranthus powellii. New Phytol 169: 251–264.
Tranel PJ, Wright TR (2002). Resistance of weeds to ALS-inhibiting herbicides: what have we learned? Weed Sci 50: 700–712.
Vila-Aiub MM, Neve P, Powles SB (2005a). Resistance cost of a cytochrome P450 herbicide metabolism mechanism but not an ACCase target site mutation in a multiple resistant Lolium rigidum population. New Phytol 167: 787–796.
Vila-Aiub MM, Neve P, Powles SB (2009). Fitness cost associated with evolved herbicide resistance alleles in plants. New Phytol 184: 751–767.
Vila-Aiub MM, Neve P, Steadman KJ, Powles SB (2005b). Ecological fitness of a multiple herbicide-resistant Lolium rigidum population: dynamics of seed germination and seedling emergence of resistant and susceptible phenotypes. J Appl Ecol 42: 288–298.
Wang T, Darmency H (1996). Comparison of growth and yield of foxtail millet (Setaria italica) resistant and susceptible to acetyl-coenzyme A carboxylase inhibiting herbicides. In: 10th Colloque International Biologie des Mauvaises Herbes de Dijon. Association Française de Protection des Plantes: Paris. pp 203–210.
Wang T, Darmency H (1997). Inheritance of sethoxydim resistance in foxtail millet, Setaria italica (L Beauv). Euphytica 94: 69–73.
Wang T, Shi Y, Li Y, Song Y, Darmency H (2010). Population growth rate of Setaria viridis in absence of herbicide: resulting yield loss in S. italica, the foxtail millet. Weed Res 50 (in press).
Wiederholt RJ, Stoltenberg DE (1996). Absence of differential fitness between giant foxtail (Setaria faberi) accessions resistant and susceptible to Acetyl-Coenzyme A Carboxylase inhibitors. Weed Sci 44: 18–24.
Yu Q, Collavo A, Zheng MQ, Owen M, Sattin M, Powles SB (2007). Diversity of acetyl-coenzyme A carboxylase mutations in resistant Lolium populations: evaluation using clethodim. Plant Physiol 145: 547–558.
Zagnikto O, Jelenska J, Tevzadze G, Haselkorn R, Gornicki P (2001). An isoleucine/leucine residue in the carboxyltransferase domain of acetyl-CoA carboxylase is critical for interaction with aryloxyphenoxypropionate and cyclohexanedione inhibitors. Proc Natl Acad Sci 98: 6617–6622.
Acknowledgements
We are especially grateful to A Fleury for field experiments and seed stock management. Research and post-doc (X. Tian) grants were provided by the Burgundy Region Council, and researcher exchanges by an AFCRST PRA contract.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, T., Picard, J., Tian, X. et al. A herbicide-resistant ACCase 1781 Setaria mutant shows higher fitness than wild type. Heredity 105, 394–400 (2010). https://doi.org/10.1038/hdy.2009.183
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hdy.2009.183
Keywords
This article is cited by
-
Germination biology of susceptible and target-site (Ile-1781-Thr) herbicide resistant short-spiked canary grass (Phalaris brachystachys) subpopulations
Acta Physiologiae Plantarum (2024)
-
Herbicide resistance status impacts the profile of non-anthocyanin polyphenolics and some phytomedical properties of edible cornflower (Centaurea cyanus L.) flowers
Scientific Reports (2023)
-
Overexpression of Polypogon fugax Type I–Like MADS-Box Gene PfAGL28 Affects Flowering Time and Pod Formation in Transgenic Arabidopsis
Plant Molecular Biology Reporter (2022)
-
No fitness cost associated with Asn-2041-Ile mutation in winter wild oat (Avena ludoviciana) seed germination under various environmental conditions
Scientific Reports (2021)
-
Transcriptomics analysis of the flowering regulatory genes involved in the herbicide resistance of Asia minor bluegrass (Polypogon fugax)
BMC Genomics (2017)