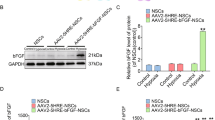
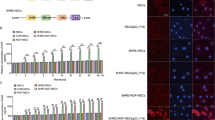
Abstract
Spinal cord injury (SCI) is an incurable disease causing an ischemic environment and functional defect, thus a new therapeutic approach is needed for SCI treatment. Vascular endothelial growth factor (VEGF) is a potent therapeutic gene to treat SCI via angiogenesis and neuroprotection, and both tissue-specific gene expression and high gene delivery efficiency are important for successful gene therapy. Here we design the hypoxia/neuron dual-specific gene expression system (pEpo-NSE) and efficient gene delivery platform can be achieved by the combination ex vivo gene therapy with erythropoietin (Epo) enhancer, neuron-specific enolase (NSE) promoter and neural stem cells (NSCs). An in vitro model, NSCs transfected with pEpo-NSE were consistently and selectively overexpressing therapeutic genes in response to neural differentiation and hypoxic conditions. Also, in SCI model, ex vivo gene therapy using pEpo-NSE system with NSCs significantly enhanced gene delivery efficiency compared with pEpo-NSE system gene therapy alone. However, microarray analysis reveals that introducing exogenous pEpo-NSE and VEGF triggers biological pathways in NSCs such as glycolysis and signaling pathways such as Ras and mitogen-activated protein kinase, leading to cell proliferation, differentiation and apoptosis. Collectively, it indicates that the pEpo-NSE gene expression system works stably in NSCs and ex vivo gene therapy using pEpo-NSE system with NSCs improves gene expression efficiency. However, exogenously introduced pEpo-NSE system has an influence on gene expression profiles in NSCs. Therefore, when we consider ex vivo gene therapy for SCI, the effects of changes in gene expression profiles in NSCs on safety should be investigated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Blesch A, Tuszynski MH . Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci 2009; 32: 41–47.
Lu K, Liang CL, Chen HJ, Chen SD, Hsu HC, Liliang PC et al. Injury severity and cell death mechanisms: effects of concomitant hypovolemic hypotension on spinal cord ischemia-reperfusion in rats. Exp Neurol 2004; 185: 120–132.
Park E, Velumian AA, Fehlings MG . The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma 2004; 21: 754–774.
Hwang DW, Kang JH, Jeong JM, Chung JK, Lee MC, Kim S et al. Noninvasive in vivo monitoring of neuronal differentiation using reporter driven by a neuronal promoter. Eur J Nucl Med Mol Imaging 2008; 35: 135–145.
Navarro V, Millecamps S, Geoffroy MC, Robert JJ, Valin A, Mallet J et al. Efficient gene transfer and long-term expression in neurons using a recombinant adenovirus with a neuron-specific promoter. Gene Therapy 1999; 6: 1884–1892.
Schmechel DE, Brightman MW, Marangos PJ . Neurons switch from non-neuronal enolase to neuron-specific enolase during differentiation. Brain Res 1980; 190: 195–214.
Oh J, You Y, Yun Y, Lee HL, Yoon do H, Lee M et al. A gene and neural stem cell therapy platform based on neuronal cell type-inducible gene overexpression. Yonsei Med J 2015; 56: 1036–1043.
Kim HA, Nam K, Lee M, Kim SW . Hypoxia/hepatoma dual specific suicide gene expression plasmid delivery using bio-reducible polymer for hepatocellular carcinoma therapy. J Control Release 2013; 171: 1–10.
Liu ML, Oh JS, An SS, Pennant WA, Kim HJ, Gwak SJ et al. Controlled nonviral gene delivery and expression using stable neural stem cell line transfected with a hypoxia-inducible gene expression system. J Gene Med 2010; 12: 990–1001.
Oh JS, An SS, Gwak SJ, Pennant WA, Kim KN, Yoon DH et al. Hypoxia-specific VEGF-expressing neural stem cells in spinal cord injury model. Neuroreport 2012; 23: 174–178.
Binley K, Iqball S, Kingsman A, Kingsman S, Naylor S . An adenoviral vector regulated by hypoxia for the treatment of ischaemic disease and cancer. Gene Therapy 1999; 6: 1721–1727.
Sun J, Wang Y, Yang J, Du D, Li Z, Wei J et al. Long-term and stable correction of uremic anemia by intramuscular injection of plasmids containing hypoxia-regulated system of erythropoietin expression. Exp Mol Med 2012; 44: 674–683.
Brockington A, Lewis C, Wharton S, Shaw PJ . Vascular endothelial growth factor and the nervous system. Neuropathol Appl Neurobiol 2004; 30: 427–446.
Silverman WF, Krum JM, Mani N, Rosenstein JM . Vascular, glial and neuronal effects of vascular endothelial growth factor in mesencephalic explant cultures. Neuroscience 1999; 90: 1529–1541.
Sondell M, Lundborg G, Kanje M . Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci 1999; 19: 5731–5740.
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002; 418: 41–49.
Stocum DL . Stem cells in CNS and cardiac regeneration. Adv Biochem Eng Biotechnol 2005; 93: 135–159.
Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med 2002; 346: 1185–1193.
Hwang, do W, Kang JH, Jeong JM, Chung JK, Lee MC et al. Noninvasive in vivo monitoring of neuronal differentiation using reporter driven by a neuronal promoter. Eur J Nucl Med Mol Imaging 2008; 35: 135–145.
Bhang SH, Kwon SH, Lee S, Kim GC, Han AM, Kwon YH et al. Enhanced neuronal differentiation of pheochromocytoma 12 cells on polydopamine-modified surface. Biochem Biophys Res Commun 2013; 430: 1294–1300.
Song KH, Li T, Owsley E, Strom S, Chiang JY . Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology 2009; 49: 297–305.
Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL . Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology 2012; 56: 1034–1043.
Hillenbrand M, Holzbach T, Matiasek K, Schlegel J, Giunta RE . Vascular endothelial growth factor gene therapy improves nerve regeneration in a model of obstetric brachial plexus palsy. Neurol Res 2015; 37: 197–203.
Han J, Calvo CF, Kang TH, Baker KL, Park JH, Parras C et al. Vascular endothelial growth factor receptor 3 controls neural stem cell activation in mice and humans. Cell Rep 2015; 10: 1158–1172.
Liu R, van Berlo JH, York AJ, Vagnozzi RJ, Maillet M, Molkentin JD . DUSP8 regulates cardiac ventricular remodeling by altering ERK1/2 signaling. Circ Res 2016; 119: 249–260.
Lang R, Hammer M, Mages J . DUSP meet immunology: dual specificity MAPK phosphatases in control of the inflammatory response. J Immunol 2006; 177: 7497–7504.
Balko JM, Schwarz LJ, Bhola NE, Kurupi R, Owens P, Miller TW et al. Activation of MAPK pathways due to DUSP4 loss promotes cancer stem cell-like phenotypes in basal-like breast cancer. Cancer Res 2013; 73: 6346–6358.
Pasca di Magliano M, Hebrok M . Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer 2003; 3: 903–911.
Ingham PW . Transducing Hedgehog: the story so far. EMBO J 1998; 17: 3505–3511.
Bosanac I, Maun HR, Scales SJ, Wen X, Lingel A, Bazan JF et al. The structure of SHH in complex with HHIP reveals a recognition role for the Shh pseudo active site in signaling. Nat Struct Mol Biol 2009; 16: 691–697.
Morelli AE, Larregina AT, Smith-Arica J, Dewey RA, Southgate TD, Ambar B et al. Neuronal and glial cell type-specific promoters within adenovirus recombinants restrict the expression of the apoptosis-inducing molecule Fas ligand to predetermined brain cell types, and abolish peripheral liver toxicity. J Gen Virol 1999; 80 (Pt 3): 571–583.
Muller FL, Colla S, Aquilanti E, Manzo VE, Genovese G, Lee J et al. Passenger deletions generate therapeutic vulnerabilities in cancer. Nature 2012; 488: 337–342.
Maxwell PH, Pugh CW, Ratcliffe PJ . Inducible operation of the erythropoietin 3' enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism. Proc Natl Acad Sci USA 1993; 90: 2423–2427.
Wang GL, Semenza GL . General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA 1993; 90: 4304–4308.
Hioki H, Kameda H, Nakamura H, Okunomiya T, Ohira K, Nakamura K et al. Efficient gene transduction of neurons by lentivirus with enhanced neuron-specific promoters. Gene Therapy 2007; 14: 872–882.
Sakimura K, Kushiya E, Takahashi Y, Suzuki Y . The structure and expression of neuron-specific enolase gene. Gene 1987; 60: 103–113.
Twyman RM, Jones EA . Sequences in the proximal 5' flanking region of the rat neuron-specific enolase (NSE) gene are sufficient for cell type-specific reporter gene expression. J Mol Neurosci 1997; 8: 63–73.
Andersen JK, Frim DM, Isacson O, Breakefield XO . Herpesvirus-mediated gene delivery into the rat brain: specificity and efficiency of the neuron-specific enolase promoter. Cell Mol Neurobiol 1993; 13: 503–515.
Duan P, Zhang Y, Han X, Liu J, Yan W, Xing Y . Effect of neuronal induction on NSE, Tau, and Oct4 promoter methylation in bone marrow mesenchymal stem cells. In Vitro Cell Dev Biol Anim 2012; 48: 251–258.
Forss-Petter S, Danielson PE, Catsicas S, Battenberg E, Price J, Nerenberg M et al. Transgenic mice expressing beta-galactosidase in mature neurons under neuron-specific enolase promoter control. Neuron 1990; 5: 187–197.
Kugler S, Meyn L, Holzmuller H, Gerhardt E, Isenmann S, Schulz JB et al. Neuron-specific expression of therapeutic proteins: evaluation of different cellular promoters in recombinant adenoviral vectors. Mol Cell Neurosci 2001; 17: 78–96.
Marangos PJ, Schmechel DE, Parma AM, Goodwin FK . Developmental profile of neuron-specific (NSE) and non-neuronal (NNE) enolase. Brain Res 1980; 190: 185–193.
Sakimura K, Kushiya E, Ogura A, Kudo Y, Katagiri T, Takahashi Y . Upstream and intron regulatory regions for expression of the rat neuron-specific enolase gene. Brain Res Mol Brain Res 1995; 28: 19–28.
Nutt SE, Chang EA, Suhr ST, Schlosser LO, Mondello SE, Moritz CT et al. Caudalized human iPSC-derived neural progenitor cells produce neurons and glia but fail to restore function in an early chronic spinal cord injury model. Exp Neurol 2013; 248: 491–503.
Choi BH, Ha Y, Ahn CH, Huang X, Kim JM, Park SR et al. A hypoxia-inducible gene expression system using erythropoietin 3' untranslated region for the gene therapy of rat spinal cord injury. Neurosci Lett 2007; 412: 118–122.
Jin H, Liu ML, Kim HA, Lee M, An S, Oh J et al. Role of the oxygen-dependent degradation domain in a hypoxia-inducible gene expression system in vascular endothelial growth factor gene therapy. Spine (Phila Pa 1976) 2009; 34: E952–E958.
Lee M, Lee ES, Kim YS, Choi BH, Park SR, Park HS et al. Ischemic injury-specific gene expression in the rat spinal cord injury model using hypoxia-inducible system. Spine (Phila Pa 1976) 2005; 30: 2729–2734.
An SS, Pennant WA, Ha Y, Oh JS, Kim HJ, Gwak SJ et al. Hypoxia-induced expression of VEGF in the organotypic spinal cord slice culture. Neuroreport 2011; 22: 55–60.
Choi UH, Ha Y, Huang X, Park SR, Chung J, Hyun DK et al. Hypoxia-inducible expression of vascular endothelial growth factor for the treatment of spinal cord injury in a rat model. J Neurosurg Spine 2007; 7: 54–60.
Lee M, Choi D, Choi MJ, Jeong JH, Kim WJ, Oh S et al. Hypoxia-inducible gene expression system using the erythropoietin enhancer and 3'-untranslated region for the VEGF gene therapy. J Control Release 2006; 115: 113–119.
Lian Jin H, Pennant WA, Hyung Lee M, Su S, Ah Kim H, Lu Liu M et al. Neural stem cells modified by a hypoxia-inducible VEGF gene expression system improve cell viability under hypoxic conditions and spinal cord injury. Spine (Phila Pa 1976) 2011; 36: 857–864.
Aiuti A, Roncarolo MG, Naldini L . Gene therapy for ADA-SCID, the first marketing approval of an ex vivo gene therapy in Europe: paving the road for the next generation of advanced therapy medicinal products. EMBO Mol Med 2017; 9: 737–740.
Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 2013; 341: 1233158.
De Ravin SS, Wu X, Moir S, Anaya-O'Brien S, Kwatemaa N, Littel P et al. Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency. Sci Transl Med 2016; 8: 335ra357.
Meneghini V, Frati G, Sala D, De Cicco S, Luciani M, Cavazzin C et al. Generation of human induced pluripotent stem cell-derived bona fide neural stem cells for ex vivo gene therapy of metachromatic leukodystrophy. Stem Cells Transl Med 2016; 6: 352–368.
Portnow J, Synold TW, Badie B, Tirughana R, Lacey SF, D'Apuzzo M et al. Neural stem cell-based anticancer gene therapy: a first-in-human study in recurrent high-grade glioma patients. Clin Cancer Res 2017; 23: 2951–2960.
Zonari E, Desantis G, Petrillo C, Boccalatte FE, Lidonnici MR, Kajaste-Rudnitski A et al. Efficient ex vivo engineering and expansion of highly purified human hematopoietic stem and progenitor cell populations for gene therapy. Stem Cell Rep 2017; 8: 977–990.
Nowakowski A, Andrzejewska A, Janowski M, Walczak P, Lukomska B . Genetic engineering of stem cells for enhanced therapy. Acta Neurobiol Exp (Wars) 2013; 73: 1–18.
Gregory CA, Prockop DJ, Spees JL . Non-hematopoietic bone marrow stem cells: molecular control of expansion and differentiation. Exp Cell Res 2005; 306: 330–335.
Dhillon AS, Hagan S, Rath O, Kolch W . MAP kinase signalling pathways in cancer. Oncogene 2007; 26: 3279–3290.
Sebolt-Leopold JS, Herrera R . Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer 2004; 4: 937–947.
Barnawi R, Al-Khaldi S, Majed Sleiman G, Sarkar A, Al-Dhfyan A, Al-Mohanna F et al. Fascin is critical for the maintenance of breast cancer stem cell pool predominantly via the activation of the notch self-renewal pathway. Stem Cells 2016; 34: 2799–2813.
Carmeliet P . VEGF as a key mediator of angiogenesis in cancer. Oncology 2005; 69 (Suppl 3): 4–10.
Alessandri G, Emanueli C, Madeddu P . Genetically engineered stem cell therapy for tissue regeneration. Ann NY Acad Sci 2004; 1015: 271–284.
Phillips MI, Tang Y . Genetic modification of stem cells for cardiac, diabetic, and hemophilia transplantation therapies. Prog Mol Biol Transl Sci 2012; 111: 285–304.
Zhang W, Liu HT . MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 2002; 12: 9–18.
Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 2003; 425: 851–856.
Cariboni A, Davidson K, Dozio E, Memi F, Schwarz Q, Stossi F et al. VEGF signalling controls GnRH neuron survival via NRP1 independently of KDR and blood vessels. Development 2011; 138: 3723–3733.
Wittko IM, Schanzer A, Kuzmichev A, Schneider FT, Shibuya M, Raab S et al. VEGFR-1 regulates adult olfactory bulb neurogenesis and migration of neural progenitors in the rostral migratory stream in vivo. J Neurosci 2009; 29: 8704–8714.
Gerber HP, Condorelli F, Park J, Ferrara N . Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem 1997; 272: 23659–23667.
Jin KL, Mao XO, Greenberg DA . Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci USA 2000; 97: 10242–10247.
Dibbens JA, Miller DL, Damert A, Risau W, Vadas MA, Goodall GJ . Hypoxic regulation of vascular endothelial growth factor mRNA stability requires the cooperation of multiple RNA elements. Mol Biol Cell 1999; 10: 907–919.
Iida K, Kawakami Y, Sone H, Suzuki H, Yatoh S, Isobe K et al. Vascular endothelial growth factor gene expression in a retinal pigmented cell is up-regulated by glucose deprivation through 3' UTR. Life Sci 2002; 71: 1607–1614.
Wenger RH . Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol 2000; 203: 1253–1263.
Acknowledgements
This work was partly supported by the Brain Korea 21 PLUS Project for Medical Science, Yonsei University and Basic Science Research Program through National Research Foundation of Korea (NRF) (No. 2015R1D1A1A02059821).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Yun, Y., Oh, J., Kim, Y. et al. Characterization of neural stem cells modified with hypoxia/neuron-specific VEGF expression system for spinal cord injury. Gene Ther 25, 27–38 (2018). https://doi.org/10.1038/gt.2017.92
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2017.92
This article is cited by
-
Gelatine nanostructured lipid carrier encapsulated FGF15 inhibits autophagy and improves recovery in spinal cord injury
Cell Death Discovery (2020)
-
Combined Method of Neuronal Cell-Inducible Vector and Valproic Acid for Enhanced Gene Expression under Hypoxic Conditions
Tissue Engineering and Regenerative Medicine (2020)