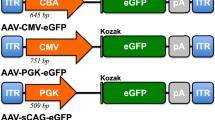
Abstract
Adeno-associated virus (AAV) vectors have been a powerful gene delivery vehicle to the retina for basic research and gene therapy. For many of these applications, achieving cell type-specific targeting and high transduction efficiency is desired. Recently, there has been increasing interest in AAV-mediated gene targeting to specific retinal bipolar cell types. A 200-bp enhancer in combination with a basal SV40 promoter has been commonly used to target transgenes into ON-type bipolar cells. In the current study, we searched for additional cis-regulatory elements in the mGluR6 gene for improving AAV-mediated transduction efficiency into retinal bipolar cells. Our results showed that the combination of the endogenous mGluR6 promoter with additional enhancers in the introns of the mGluR6 gene markedly enhanced AAV transduction efficiency as well as made the targeting more selective for rod bipolar cells in mice. Furthermore, the AAV vectors with the improved promoter could target to ON bipolar cells with robust transduction efficiency in the parafovea and the far peripheral retina of marmoset monkeys. The improved mGluR6 promoter constructs could provide a valuable tool for genetic manipulation in rod bipolar cells in mice and facilitate clinical applications for ON bipolar cell-based gene therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Zhu Y, Xu J, Hauswirth WW, DeVries SH . Genetically targeted binary labeling of retinal neurons. J Neurosci 2014; 34: 7845–7861.
Maguire AM, Simonelli F, Pierce EA, Pugh Jr EN, Mingozzi F, Bennicelli J et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med 2008; 358: 2240–2248.
Boye SE, Boye SL, Lewin AS, Hauswirth WW . A comprehensive review of retinal gene therapy. Mol Ther 2013; 21: 509–519.
Trapani I, Puppo A, Auricchio A . Vector platforms for gene therapy of inherited retinopathies. Prog Retin Eye Res 2014; 43: 108–128.
Vandenberghe LH, Auricchio A . Novel adeno-associated viral vectors for retinal gene therapy. Gene Therapy 2012; 19: 162–168.
Euler T, Haverkamp S, Schubert T, Baden T . Retinal bipolar cells: elementary building blocks of vision. Nat Rev Neurosci 2014; 15: 507–519.
Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM et al. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 2006; 50: 23–33.
Lagali PS, Balya D, Awatramani GB, Munch TA, Kim DS, Busskamp V et al. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci 2008; 11: 667–675.
Busskamp V, Picaud S, Sahel JA, Roska B . Optogenetic therapy for retinitis pigmentosa. Gene Therapy 2012; 19: 169–175.
Pan Z-H, Lu Q, Bi A, Dizhoor AM, Abrams GW . Optogenetic approaches to restoring vision. Annu Rev Vis Sci 2015; 1: 185–210.
Ueda Y, Iwakabe H, Masu M, Suzuki M, Nakanishi S . The mGluR6 5’ upstream transgene sequence directs a cell-specific and developmentally regulated expression in retinal rod and ON-type cone bipolar cells. J Neurosci 1997; 17: 3014–3023.
Morgan JL, Dhingra A, Vardi N, Wong RO . Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat Neurosci 2006; 9: 85–92.
Dhingra A, Sulaiman P, Xu Y, Fina ME, Veh RW, Vardi N . Probing neurochemical structure and function of retinal ON bipolar cells with a transgenic mouse. J Comp Neurol 2008; 510: 484–496.
Kim DS, Matsuda T, Cepko CL . A core paired-type and POU homeodomain-containing transcription factor program drives retinal bipolar cell gene expression. J Neurosci 2008; 28: 7748–7764.
Doroudchi MM, Greenberg KP, Liu J, Silka KA, Boyden ES, Lockridge JA et al. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol Ther 2011; 19: 1220–1229.
Cronin T, Vandenberghe LH, Hantz P, Juttner J, Reimann A, Kacsó AE et al. Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter. EMBO Mol Med 2014; 6: 1175–1190.
Mace E, Caplette R, Marre O, Sengupta A, Chaffiol A, Barbe P et al. Targeting channelrhodopsin-2 to ON-bipolar cells with vitreally administered AAV restores ON and OFF visual responses in blind mice. Mol Ther 2015; 23: 7–16.
Dalkara D, Kolstad KD, Caporale N, Visel M, Klimczak RR, Schaffer DV et al. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol Ther 2009; 17: 2096–2102.
Petrs-Silva H, Dinculescu A, Li Q, Deng WT, Pang JJ, Min SH et al. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol Ther 2011; 19: 293–301.
Lu Q, Ivanova E, Ganjawala HT, Pan Z-H . Cre-mediated recombination efficiency and transgene expression patterns of three retinal bipolar cell-expressing Cre transgenic mouse lines. Mol Vis 2013; 19: 1310–1320.
Butler JE, Kadonaga JT . The RNA polymerase II core promoter: a key component in the regulation of gene expression. Genes Dev 2002; 16: 2583–2592.
Papadakis ED, Nicklin SA, Baker AH, White SJ . Promoters and control elements: designing expression cassettes for gene therapy. Curr Gene Ther 2004; 4: 89–113.
Pennacchio LA, Bickmore W, Dean A, Nobrega MA, Bejerano G . Enhancers: five essential questions. Nat Rev Genet 2013; 14: 288–295.
Visel A, Blow MJ, Li Z, Zhang T, Akiyama JA, Holt A et al. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature 2009; 457: 854–858.
Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, Pang JJ et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther 2009; 17: 463–471.
Greferath U, Grünert U, Wässle H . Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol 1990; 301: 433–442.
Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med 2013; 5: 189ra76.
Huang L, Max M, Margolskee RF, Su H, Masland RH, Euler T . G protein subunit Gγ13 is coexpressed with Gαo, Gβ3, and Gβ4 in retinal ON bipolar cells. J Comp Neurol 2003; 455: 1–10.
Chan TL, Martin PR, Clunas N, Grünert U . Bipolar cell diversity in the primate retina: morphologic and immunocytochemical analysis of a new world monkey, the marmoset Callithrix jacchus. J Comp Neurol 2001; 437: 219–239.
Weltzien F, Percival KA, Martin PR, Grünert U . Analysis of bipolar and amacrine populations in marmoset retina. J Comp Neurol 2015; 523: 313–334.
Surace EM, Auricchio A . Versatility of AAV vectors for retinal gene transfer. Vis Res 2008; 48: 353–359.
de Leeuw CN, Dyka FM, Boye SL, Laprise S, Zhou M, Chou AY et al. Targeted CNS delivery using human MiniPromoters and demonstrated compatibility with adeno-associated viral vectors. Mol Ther Methods Clin Dev 2014; 1: 5.
Scalabrino ML, Boye SL, Fransen KM, Noel JM, Dyka FM, Min SH et al. Intravitreal delivery of a novel AAV vector targets ON bipolar cells and restores visual function in a mouse model of complete congenital stationary night blindness. Hum Mol Genet 2015; 24: 6229–6239.
Wässle H, Puller C, Müller F, Haverkamp S . Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci 2009; 29: 106–117.
Strettoi E, Pignatelli V . Modifications of retinal neurons in a mouse model of retinitis pigmentosa. Proc Natl Acad Sci USA 2000; 97: 11020–11025.
Hibino H, Tani K, Ikebuchi K, Wu MS, Sugiyama H, Nakazaki Y et al. The common marmoset as a target preclinical primate model for cytokine and gene therapy studies. Blood 1999; 93: 2839–2848.
t’Hart BA, Vervoordeldonk M, Heeney JL, Tak PP . Gene therapy in nonhuman primate models of human autoimmune disease. Gene Therapy 2003; 10: 890–901.
Ivanova E, Hwang GS, Pan ZH, Troilo D . Evaluation of AAV-mediated expression of Chop2-GFP in the marmoset retina. Investig Ophthalmol Vis Sci 2010; 51: 5288–5296.
Baba Y, Satoh S, Otsu M, Sasaki E, Okada T, Watanabe S . In vitro cell subtype-specific transduction of adeno-associated virus in mouse and marmoset retinal explant culture. Biochimie 2012; 94: 2716–2822.
Troilo D, Howland HC, Judge SJ . Visual optics and retinal cone topography in the common marmoset (Callithrix jacchus. Vision Res 1993; 33: 1301–1310.
Goodchild AK, Ghosh KK, Martin PR . Comparison of photoreceptor spatial density and ganglion cell morphology in the retina of human, macaque monkey, cat, and the marmoset Callithrix jacchus. J Comp Neurol 1996; 366: 55–75.
Wilder HD, Grunert U, Lee BB, Martin PR . Topography of ganglion cells and photoreceptors in the retina of a New World monkey: the marmoset Callithrix jacchus. Vis Neurosci 1996; 13: 335–352.
Hendrickson A, Troilo D, Djajadi H, Possin D, Springer A . Expression of synaptic and phototransduction markers during photoreceptor development in the marmoset monkey Callithrix jacchus. J Comp Neurol 2009; 512: 218–231.
Hendrickson A, Troilo D, Possin D, Springer A . Development of the neural retina and its vasculature in the marmoset Callithrix jacchus. J Comp Neurol 2006; 497: 270–286.
Cehajic-Kapetanovic J, Eleftheriou C, Allen AE, Milosavljevic N, Pienaar A, Bedford R et al. Restoration of vision with ectopic expression of human rod opsin. Curr Biol 2015; 25: 2111–2122.
Gaub BM, Berry MH, Holt AE, Reiner A, Kienzler MA, Dolgova N et al. Restoration of visual function by expression of a light-gated mammalian ion channel in retinal ganglion cells or ON-bipolar cells. Proc Natl Acad Sci USA 2014; 111: E5574–E5583.
Gaub BM, Berry MH, Holt AE, Isacoff EY, Flannery JG . Optogenetic vision restoration using rhodopsin for enhanced sensitivity. Mol Ther 2015; 23: 1562–1571.
Yin L, Greenberg K, Hunter JJ, Dalkara D, Kolstad KD, Masella BD et al. Intravitreal injection of AAV2 transduces macaque inner retina. Investig Ophthalmol Vis Sci 2011; 52: 2775–2783.
Kay CN, Ryals RC, Aslanidi GV, Min SH, Ruan Q, Sun J et al. Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS One 2013; 8: e62097.
Aslanidi GV, Rivers AE, Ortiz L, Song L, Ling C, Govindasamy L et al. Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: the final threshold? PLoS One 2013; 8: e59142.
Acknowledgements
This work was supported by National Institutes of Health (NIH) Grant EY17130 (to Z-HP), Core Grant EY04068 to Department of Anatomy and Cell Biology at Wayne State University, Dryer Foundation, the Ligon Research Center of Vision and Research to Prevent Blindness to Department of Ophthalmology at Wayne State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Q Lu, TH Ganjawala, JG Cheng and Z-H Pan are inventors of the improved promoter constructs. Z-H Pan services as Scientific Advisor to RetroSense Therapeutics.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Lu, Q., Ganjawala, T., Ivanova, E. et al. AAV-mediated transduction and targeting of retinal bipolar cells with improved mGluR6 promoters in rodents and primates. Gene Ther 23, 680–689 (2016). https://doi.org/10.1038/gt.2016.42
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2016.42
This article is cited by
-
Improving adeno-associated viral (AAV) vector-mediated transgene expression in retinal ganglion cells: comparison of five promoters
Gene Therapy (2023)
-
Towards translational optogenetics
Nature Biomedical Engineering (2022)
-
Bipolar cell targeted optogenetic gene therapy restores parallel retinal signaling and high-level vision in the degenerated retina
Communications Biology (2022)
-
A 2020 vision of ocular gene therapy
Gene Therapy (2021)
-
Gene therapy in the CNS—one size does not fit all
Gene Therapy (2021)