Abstract
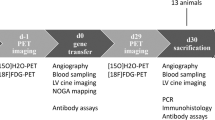
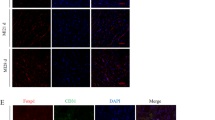
We aimed to control the gene expression of vascular endothelial growth factor (VEGF) and angiopoietin-1 (Ang-1) in the ischemic heart to explore the feasibility of sequential, timely and controlled multigene expression as a means of improving therapeutic angiogenesis in vivo. Adult rabbit myocardial infarction models were surgically established (n=120). Hypoxia-inducible factor-1α-hypoxic response element (HIF1α-HRE) and Tet (tetracycline)-On advanced gene control systems were reconstructed for controlled expression of the human VEGF165 (hVEGF165) and Ang-1 genes, respectively. Recombinant adeno-associated viruses (rAAV)-9HRE-hVEGF165 and rAAV-TRE-Tight-Ang-1 were delivered into the ischemic myocardium for 12 weeks. Reverse transcription-polymerase chain reaction, western blotting and immunofluorescence staining were used to detect gene and protein expression. Vessel functionality, vascular permeability and animal cardiac function were also evaluated. Under the control of the HIF1α-HRE and Tet-On gene control systems, the expression of the exogenous hVEGF165 and Ang-1 genes was consistent in the ischemia control. In the sequential group, we found that the number of functional vessels with a larger diameter and more vascular branches was increased, and vascular permeability was significantly reduced. In addition, animal heart function was significantly improved compared with the non-sequential and hVEGF165- or Ang-1-only groups (P<0.05, P<0.05, respectively). Sequential, timely and controlled expression of the hVEGF165 and Ang-1 genes in vivo is a new therapeutic angiogenesis strategy that can effectively promote functional vessel regeneration and can improve cardiac function in ischemic heart disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Simons M . Angiogenesis: where do we stand now? Circulation 2005; 111: 1556–1566.
Siddiqui J, Fischer H, Widegren U, Grinnemo KH, Hao X, Mansson-Broberg A et al. Depressed expression of angiogenic growth factors in the subacute phase of myocardial ischemia: a mechanism behind the remodeling plateau? Coron Artery Dis 2010; 21: 65–71.
Matsunaga T, Warltier DC, Tessmer J, Weihrauch D, Simons M, Chilian WM . Expression of VEGF and angiopoietins-1 and -2 during ischemia-induced coronary angiogenesis. Am J Physiol Heart Circ Physiol 2003; 285: H352–H358.
Gavard J, Patel V, Gutkind JS . Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell 2008; 14: 25–36.
Konstantin G, G Guillem, A Annika, B Christer . Endothelial–mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 2009; 29: 630–638.
Markus T, Hellmut GA . The role of the angiopoietins in vascular morphogenesis. Angiogenesis 2009; 12: 125–137.
Salmon AH, Neal CR, Sage LM, Glass CA, Harper SJ, Bates DO . Angiopoietin-1 alters microvascular permeability coefficients in vivo via modification of endothelial glycocalyx. Cardiovasc Res 2009; 83: 24–33.
Lee KW, Lip GY, Blann AD . Plasma angiopoietin-1, angiopoietin-2, angiopoietin receptor tie-2, and vascular endothelial growth factor levels in acute coronary syndromes. Circulation 2004; 110: 2355–2360.
Zhang H, Dong HY, Jiang B, Wang Z, Chen R, Zhang Z et al. Hypoxic response elements and Tet-On advanced double-controlled systems regulate hVEGF165 and angiopoietin-1 gene expression in vitro. J Biomed Res 2011; 3: 204–212.
Carmeliet P, De Smet F, Loges S, Mazzone M . Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way. Nat Rev Clin Oncol 2009; 6: 315–326.
Warren CM, Iruela-Arispea ML . Signaling circuitry in vascular morphogenesis. Curr Opin Hematol 2010; 17: 213–218.
Shyu K-G, Chang H, MI Jeffrey . Synergistic effect of angiopoietin-1 and vascular endothelial growth factor on neoangiogenesis in hypercholesterolemic rabbit model with acute hindlimb ischemia. Life Sci 2003; 73: 563–579.
Zhou YF, Stabile E, Walker J, Shou M, Baffour R, Yu Z et al. Effects of gene delivery on collateral development in chronic hypoperfusion: diverse effects of angiopoietin-1 versus vascular endothelial growth factor. Am Coll Cardiol 2004; 8: 897–903.
Carmeliet P . Angiogenesis in life, disease and medicine. Nature 2005; 438: 932–936.
Thurston G, Wang Q, Baffert F, Rudge J, Papadopoulos N, Jean-Guillaume D et al. Angiopoietin 1 causes vessel enlargement, without angiogenic sprouting, during a critical developmental period. Development 2005; 132: 3317–3326.
Yana I, Sagara H, Takaki S, Takatsu K, Nakamura K, Nakao K et al. Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J Cell Sci 2007; 120: 1607–1614.
van Hinsbergh VWM, Koolwijk P . Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res 2008; 78: 203–212.
London NR, Whitehead KJ, Li DY . Endogenous endothelial cell signaling systems maintain vascular stability. Angiogenesis 2009; 12: 149–158.
Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvis A, Abramsson A et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 2003; 161: 1163–1177.
Betsholtz C, Shima D . Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev 2002; 16: 2684–2698.
Ye L, Haider HKh, Jiang S, Tan RS, Ge R, Law PK et al. Improved angiogenic response in pig heart following ischemic injury using human skeletal myoblast simultaneously expressing VEGF165 and angiopoietin-1. Eur J Heart Fail 2007; 9: 15–22.
Niagara MI, Haider HK, Ye L, Koh VS, Lim YT, Poh KK et al. Autologous skeletal myoblasts transduced with a new adenoviral bicistronic vector for treatment of hind limb ischemia. J Vasc Surg 2004; 40: 774–785.
Vera Janavel G, Crottogini A, Cabeza Meckert P, Cuniberti L, Mele A, Papouchado M et al. Plasmid-mediated VEGF gene transfer induces cardiomyogenesis and reduces myocardial infarct size in sheep. Gene Therapy 2006; 13: 1133–1142.
Ferrarini M, Arsic N, Recchia FA, Zentilin L, Zacchigna S, Xu X et al. Adeno-associated virus-mediated transduction of VEGF165 improves cardiac tissue viability and functional recovery after permanent coronary occlusion in conscious dogs. Circ Res 2006; 98: 954–961.
Dong HY, Xu ZW, Zhang ZM, Yu HL, Xu XH . Mobilization of bone marrow-derived Nkx2-5(+) cardiac progenitor cells under condition of acute myocardial ischemia. Acta Physiol Sin 2009; 61: 185–193.
Jiang B, Dong HY, Zhang ZM, Wang W, Zhang YQ, Xu XH . Hypoxic response elements control expression of human vascular endothelial growth factor165 genes transferred to ischemia myocardium in vivo and in vitro. J Gene Med 2007; 9: 788–796.
Favre D, Blouin V, Provost N, Spisek R, Porrot F, Bohl D et al. Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J Virol 2002; 76: 11605–11611.
Dong HY, Wang Q, Zhang YQ, Jiang B, Xu XH, Zhang ZM . Angiogenesis induced by hVEGF165 gene controlled by hypoxic response elements in rabbit ischemia myocardium. Exp Biol Med 2009; 234: 1417–1424.
Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009; 324: 1710–1713.
Rutanen J, Rissanen TT, Markkanen JE, Gruchala M, Silvennoinen P, Kivelä A et al. Adenoviral catheter-mediated intramyocardial gene transfer using the mature form of vascular endothelial growth factor-D induces transmural angiogenesis in porcine heart. Circulation 2004; 109: 1029–1035.
Emanueli C, Grady EF, Madeddu P, Figin M, Bunnett NW, Parisi D et al. Acute ACE inhibition causes plasma extravasation in mice that is mediated by bradykinin and substance P. Hypertension 1998; 31: 1299–1304.
Acknowledgements
This work was supported by a Grant from National Natural Science Foundation of China (30672081).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, H., Yuan, Y., Wang, Z. et al. Sequential, timely and controlled expression of hVEGF165 and Ang-1 effectively improves functional angiogenesis and cardiac function in vivo. Gene Ther 20, 893–900 (2013). https://doi.org/10.1038/gt.2013.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2013.12
Keywords
This article is cited by
-
PEDF and PEDF-derived peptide 44mer protect cardiomyocytes against hypoxia-induced apoptosis and necroptosis via anti-oxidative effect
Scientific Reports (2014)
-
Antidepressant-induced vascular dynamics in the hippocampus of adult mouse brain
Cell and Tissue Research (2014)