Abstract
Purpose:
Very-long-chain acyl-CoA dehydrogenase (VLCAD) deficiency (VLCADD) is an inherited disorder of mitochondrial long-chain fatty acid β-oxidation (LC-FAO) and is included in many newborn screening (NBS) programs worldwide. Patients may present with hypoketotic hypoglycemia, cardiomyopathy, and/or myopathy, but clinical severity varies widely and the clinical outcome is unpredictable. We investigated predictive markers that may determine clinical severity.
Methods:
We developed a clinical severity score (CSS), which was determined for 13 Dutch patients with VLCADD, all of whom were diagnosed before the introduction of VLCADD in NBS to prevent bias from early diagnosis. In cultured skin fibroblasts from these patients, we measured LC-FAO flux (the rate of oleate oxidation), VLCAD activity, and acylcarnitine profiles following palmitate loading.
Results:
The strongest correlation (r = 0.93; P < 0.0001) was observed between LC-FAO flux and the CSS. VLCAD activity measurement and the C14/C16-to-acylcarnitine ratio correlated much less. A median LC-FAO flux of 6% of control values (range 5.6–6.8%) was associated with cardiomyopathy (P < 0.01), and 32.4% (range 5.6–50.5%) was associated with myopathy (P < 0.05).
Conclusion:
Our results demonstrate a very strong correlation between LC-FAO flux in fibroblasts and the clinical severity of VLCADD. LC-FAO flux measurements may thus predict whether patients are likely to develop symptoms.
Genet Med 17 12, 989–994.
Similar content being viewed by others
Introduction
Very-long-chain acyl-CoA dehydrogenase (VLCAD) deficiency (VLCADD) (OMIM ID for VLCAD deficiency: 201475), an autosomal-recessive inherited disorder of mitochondrial long-chain fatty acid β-oxidation (LC-FAO), is caused by mutations in the ACADVL gene. Patients may present with a variety of clinical signs and symptoms, including hypoketotic hypoglycemia, hepatomegaly, cardiomyopathy, and myopathy. These symptoms can be triggered by illness, fever, exercise, and fasting.1,2,3,4 VLCADD is currently included in many newborn screening (NBS) programs all over the world.5,6 Most newborns with VLCADD identified by NBS are asymptomatic at the time of referral.7 Because these patients are considered to be at risk of potentially life-threatening symptoms, parents often get dietary advice, including strict avoidance of fasting.8 However, since the introduction of VLCADD in NBS panels, it has become clear that a significant number of newborns with VLCADD actually have a very low risk for metabolic decompensation and may even remain fully asymptomatic if left untreated.9,10,11 Unfortunately, there is currently no reliable method to assess the expected phenotypic severity at the time of diagnosis through NBS. The available literature is biased by reports of symptomatic patients; consequently, genotype–phenotype correlation studies concern more severe presentations. These studies show that nonsense mutations in the encoding gene (ACADVL) result in a severe and early presentation of cardiomyopathy,12,13,14 but the more frequent missense mutations are associated with both severe or attenuated presentations.10
Functional tests, including determination of the residual activity of the VLCAD enzyme,10,15 have been used to test the effects of various mutations on LC-FAO activity. VLCAD enzyme activity measurement as the sole functional readout is disadvantageous because it is not well suited to estimating the influence of genetic variations in potential compensatory enzymes (e.g., MCAD15 or ACAD9 (ref. 16)). For this reason, we also performed flux studies and determined the rate of oleate oxidation (C18:1) in whole cells (LC-FAO flux). In addition, we performed acylcarnitine profiling after loading cultured skin fibroblasts with labeled palmitate (in vitro probe assay).15,17,18,19,20,21
To identify potential predictors of disease severity in VLCADD, we studied clinical severity in 13 patients by applying a new VLCADD clinical severity score (CSS). Patients identified by NBS were excluded because starting treatment early affects the natural history and masks the clinical severity prediction. In our patients diagnosed before NBS, we found a strong correlation between the CSS and LC-FAO flux, whereas VLCAD enzyme activity and acylcarnitine measurements after palmitate loading were less predictive.
Materials and Methods
The study was approved by the medical ethics committee of the University Medical Centre Utrecht (METC 10–430/C). All patients gave written informed consent for participation in this study.
CSS algorithm
We used the objectively verifiable signs and symptoms most frequently reported in the literature12,22,23 (Supplementary Table S1 online) to develop a CSS. The CSS encompasses the following criteria: (i) hypoglycemia (i.e., documented glucose <2.5 mmol/l); (ii) cardiomyopathy and/or arrhythmia (i.e., documented abnormal results on echocardiography (with left or right ventricular wall thickness of at least one segment >2 SD, corrected for age)) and/or electrocardiogram abnormalities; and (iii) myopathy (i.e., documented creatine kinase concentrations >250 U/L (reference values 70–170 U/L)), as well as at least two of the following clinically relevant symptoms: myoglobulinuria, myalgia, exercise intolerance, muscle weakness (medical research council grade 4 or less), and/or frequent fatigue.
A score of one point was given for each criterion present, resulting in a CSS of 0, 1, 2, or 3.
Hepatomegaly was not included in the CSS because in most patients no standardized measurement of liver size had been performed either by ultrasound or magnetic resonance imaging.
Assays in fibroblasts
Fibroblasts of all patients and controls were cultured in HAM F-10 in parallel, and all tests were performed on all cell lines on the same day to prevent interassay variability. In addition, fibroblasts were cultured for 2 weeks at 30, 37, and 40 °C before the biochemical assays were performed.
LC-FAO flux analysis
LC-FAO flux was determined by measuring the production of radiolabeled H2O from [9,10-3H(N)]-oleic acid, as described previously.18,21,24 Measurements were made at 37 °C in quadruplicate, and oxidation rates were expressed as nanomoles of fatty acid oxidized per hour per milligram of cellular protein.
VLCAD activity measurements
VLCAD activity in fibroblasts was determined using C16:0-CoA as the substrate and ferrocenium hexafluorophosphate as the electron acceptor, followed by ultraperformance liquid chromatography to separate the different acyl-CoA species, as described previously.15,25 Measurements were made at 37 °C and in duplicate.
Acylcarnitine profiling
Fibroblasts were cultured in 12-well plates and incubated for 96 hours at 37 °C, 5% CO2, in minimum essential medium with a 1% mixture of penicillin, streptomycin, and fungizone (Gibco, Grand Island, NY). Furthermore, 0.4 mmol/l L-carnitine, 0.4% bovine serum albumin, and 100 µmol/l [U-13C]palmitate were added to the medium. After 96 hours, the medium was removed from the cells, followed by deproteinization using acetonitrile, with subsequent analysis of the acylcarnitines by tandem mass spectrometry.17,26 These measurements were done in duplicate.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA). We used the Pearson correlation coefficient to test correlation between CSS and functional tests. An unpaired t-test was used to test differences between the two groups with and without myopathy, cardiomyopathy, and hypoglycemia. P values <0.05 (indicated with *) and <0.01 (indicated with **) were considered statistically significant, as appropriate.
Results
Patients characteristics and CSS
Thirteen patients diagnosed with VLCADD in the Netherlands between January 1972 and January 2007, before the introduction of VLCADD in the NBS program, were included in this study. Patient characteristics are summarized in Table 1 . Two patients had a CSS of 0, four patients had a score of 1, three had a score of 2, and four had a CSS of 3 points ( Table 1 ).
Genotype
The genotypes of all patients are presented in Table 2 . In total, 14 different mutations were detected, including 5 mutations that were not reported before. The most common mutations were c.104delC, p.Pro35LeufsX26 (n = 3, five alleles), and c.848T>C, p.Val283Ala (n = 4, four alleles).
Functional assays
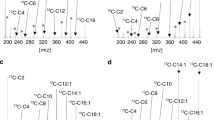
Fibroblasts from all 13 VLCADD patients were available. Patient identification numbers (PIDs) were assigned based on the results of LC-FAO flux analysis at 37 °C; PID 1 had the lowest and PID 13 the highest LC-FAO flux. To evaluate the effect of temperature on VLCAD activity and on LC-FAO flux, we cultured fibroblasts for a period of 2 weeks at 30, 37, and 40 °C. Culturing at 30 °C allows more efficient protein folding and may stimulate LC-FAO flux, whereas 40 °C decreases stability and mimics a situation in which cells and thus fatty acid β-oxidation enzymes have to deal with the stress imposed by increased temperatures in vitro. LC-FAO flux was very low in PID 1–4 (range 5.6–6.6% of controls) regardless of temperature, and flux was higher in PID 5–13 (range 32.4–93.0% of controls) ( Figure 1a ). Interestingly, LC-FAO flux improved in virtually all patients at 30 °C. In some, activity increased more than 50% compared with LC-FAO flux in control fibroblasts cultured at 37 °C (PID 5, 6, 8, and 9). At 40 °C, LC-FAO flux was considerably lower in both control and patient fibroblasts. In some patients, LC-FAO flux decreased by as much as 40% compared with cells cultured at 37 °C (PID 6, 7, and 9–13) ( Figure 1a ).
Long-chain fatty acid β-oxidation (LC-FAO) flux, very-long-chain acyl-CoA dehydrogenase (VLCAD) activity, and acylcarnitine assay. (a) LC-FAO flux ([9,10-3H(N)]-oleic acid oxidation rate) and (b) VLCAD activity (with palmitoyl-CoA as substrate) in cells from 13 patients were measured after being cultured for 2 weeks at 30, 37, and 40 °C. Concentrations of various (long-chain) acylcarnitines after 96 hours of incubation with [U-13C]palmitate at 37 °C were plotted, including (c) C12,C14,C16, (d) C2, (e) and their corresponding ratios: C12/C16, C14/C16, and C14/C2. Patient identification numbers (PIDs) were assigned based on the results of LC-FAO flux analysis at 37 °C. PID 1 had the lowest and PID 13 the highest LC-FAO flux.
VLCAD enzyme activity was markedly reduced in all patients at 37 °C (<16% of control fibroblasts) and not detectable at 40 °C. At 30 °C, VLCAD activity increased up to 18–27% in PID 5, 6, and 8–13 ( Figure 1b ), whereas VLCAD activity remained undetectable in PID 1–4.
Strikingly, LC-FAO flux was relatively high even with low residual VLCAD enzymatic activity. That a VLCAD residual activity of 20% seems to be sufficient to allow normal rates of whole-cell oleate oxidation is noteworthy (Supplementary Figure S1 online).
Acylcarnitine profiling shows that several patients displayed a four- to fivefold accumulation of C14 and a two- to threefold increase in C16 level (PID 1–4) ( Figure 1c ). The level of C14 was lower in PID 5–13 compared with PID 1–4. In patients 1, 2, and 4, greater than a twofold decrease in C2 levels was found compared with other patients ( Figure 1d ). The ratio of C14 to C2 was particularly high in PID 1–4, in line with their almost full block of LC-FAO flux. By contrast, C12/C16 was decreased up to threefold in PID 1–4 compared with other patients ( Figure 1e ; Supplementary Table S2 online).
Correlation of CSS with functional studies
We investigated the correlation between the various fibroblast tests and the CSS. As shown in Figure 2 , a strong and highly significant correlation (Pearson correlation coefficient −0.93; P < 0.0001) between LC-FAO flux and CSS was found ( Figure 2a ). Residual VLCAD enzymatic activity measurement and the C14/C16-to-acylcarnitine ratio also correlated significantly with the CSS, but to a lesser extent (VLCAD: Pearson r = −0.78, P = 0.0014 ( Figure 2b ); acylcarnitines: r = 0.75, P = 0.003 (Supplementary Figure S2 online)). In particular, LC-FAO flux has more distinctive power and accuracy in the lower activity range.
Correlation between clinical severity and biochemical parameters in VLCADD patient fibroblasts. (a) Long-chain fatty acid β-oxidation (LC-FAO) flux versus clinical severity score and (b) very-long-chain acyl-CoA dehydrogenase (VLCAD) activity versus clinical severity score. r, Pearson correlation coefficient.
We then determined the level of LC-FAO flux that is associated with the presentation of the three main symptoms. We observed that patients with cardiomyopathy have a median LC-FAO flux of 6.0% of control values (range 5.6–6.6%) compared with 39.4% (range 32.4–93.0%) in patients without cardiomyopathy (P < 0.01) ( Figure 3a ). Patients with myopathy had a median LC-FAO flux of 32.4% (range 5.6–50.5%) compared with 60.6% (range 35.3–93.0%) in patients without myopathy (P < 0.05) ( Figure 3b ). Patients with hypoglycemia had a median LC-FAO flux of 32.9% (range 5.6–52.0%), compared with 50.4% (range 6.0–93.0%) in patients who did not experience hypoglycemia (P = 0.22) ( Figure 3c ).
Discussion
Since the introduction of VLCADD in NBS programs, an increasing number of asymptomatic individuals has been identified.10,11,27 Our study aimed to develop a tool that predicts the clinical phenotype in individuals with VLCADD detected by NBS. In this article we show a strong correlation between LC-FAO flux in cultured skin fibroblasts and the clinical severity of the phenotype in patients with VLCADD diagnosed before introduction of VLCADD in the Dutch NBS program. We performed all tests in all cell lines on the same day to prevent interassay variability. We show that, compared with the other assays, LC-FAO flux is a particularly accurate measure in the lower range of residual activity. In addition, LC-FAO flux measurement in fibroblasts cultured at 37 °C has the most distinctive power: it ranges between 6 and 93% of the control value, compared with 0–16% for the VLCAD assay. Hence we suggest that LC-FAO flux analysis of cultured fibroblasts is a useful tool for predicting the risk of developing symptoms.
We demonstrate that LC-FAO flux in fibroblasts at 37 °C is the most distinctive way to map the capacity of a patient with VLCADD to break down long-chain fatty acids. We used a protocol that has been described before,18 widely used, and proved reproducible.
Remarkably, LC-FAO flux was much higher than expected on the basis of VLCAD activity alone. For instance, even at a residual VLCAD activity of 20%, the flux through the LC-FAO system is equal to that among controls. This is also observed in fibroblasts of patients with carnitine palmitoyltransferase 2 deficiency,28 which is fully in line with our clinical findings; the patients with the highest residual VLCAD activity—6 and 18% for PID 12 and 13, respectively—do not display symptoms.
Interestingly, despite a residual VLCAD activity of less than 1%, patients are still able to maintain a LC-FAO flux of ~6%. This strongly suggests that the residual activity is independent of VLCAD. MCAD, ACAD9, and/or peroxisomal fatty acid oxidation may likely be responsible for this activity.15,16,29,30
In addition to measuring LC-FAO flux at 37 °C, we also studied flux in cultured fibroblasts at different temperatures, which revealed that cell lines from some patients show a significant decrease in LC-FAO flux when cultured at 40 °C. Indeed, in patients with a LC-FAO flux of more than 6%, a 40% decrease in LC-FAO flux was observed at 40 °C (PID 6, 7, and 11). Hence, some patients with relatively high residual enzyme activity and no or only very mild symptoms under normal conditions may develop severe symptoms during a situation in which fatty acid oxidation enzymes have to deal with the stress imposed by increased temperatures because of the drop in LC-FAO flux.
Fibroblasts of some patients (PID 5–12) show a marked increase in LC-FAO flux when cultured at 30 °C. This indicates that VLCAD in these cell lines is more stable at 30 °C, suggesting that protein misfolding and subsequent rapid degradation play a role in the loss of activity at higher temperatures. The use of chemical chaperones for VLCAD might therefore have a beneficial effect by stabilizing the mutant protein and enhance protein folding at 37 °C.31
Our data suggest that functional assays in fibroblasts are better predictors of clinical severity than specific genotypes. Missense mutations often cause variable clinical phenotypes. For example, clinical phenotypes of patients with c.643T>C (p.Cys215Arg) resulted in a very severe phenotype. Patients with one c.848T>C (p.Val283Ala) allele ranged from expected mild10,12 to quite severe phenotypes (PID 5 and 9; Tables 1 and 2 ).
A potential limitation of our study is its relatively small sample size. This could have resulted in inclusion bias, in particular, overrepresentation of certain clinical phenotypes. We think this is unlikely because we included Dutch patients who were identified before NBS and who represent the full range of clinical phenotypes, from mild to severe. Although additional studies are needed to corroborate our findings, including only treatment-naïve patients is very important because therapy interferes with the natural history.
In summary, our biochemical and clinical data demonstrate that LC-FAO flux has a strong correlation with clinical severity in patients with VLCADD. In addition, the LC-FAO flux of some patients decreased by 40% when cells were cultured at 40 °C. Measurement of LC-FAO flux in asymptomatic individuals with VLCADD might therefore be a useful tool to predict whether patients are likely to develop symptoms in a situation of metabolic stress. Treatment could thereafter be adjusted accordingly.
Disclosure
The authors declare no conflict of interest.
References
Bonnet D, Martin D, Pascale De Lonlay, et al. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation 1999;100:2248–2253.
Rinaldo P, Matern D, Bennett MJ. Fatty acid oxidation disorders. Annu Rev Physiol 2002;64:477–502.
Spiekerkoetter U, Mayatepek E. Update on mitochondrial fatty acid oxidation disorders. J Inherit Metab Dis 2010;33:467–468.
Wanders RJ, Vreken P, den Boer ME, Wijburg FA, van Gennip AH, IJlst L. Disorders of mitochondrial fatty acyl-CoA beta-oxidation. J Inherit Metab Dis 1999;22:442–487.
Lindner M, Hoffmann GF, Matern D. Newborn screening for disorders of fatty-acid oxidation: experience and recommendations from an expert meeting. J Inherit Metab Dis 2010;33:521–526.
Loeber JG, Burgard P, Cornel MC, et al. Newborn screening programmes in Europe; arguments and efforts regarding harmonization. Part 1. From blood spot to screening result. J Inherit Metab Dis 2012;35:603–611.
Spiekerkoetter U, Sun B, Zytkovicz T, Wanders R, Strauss AW, Wendel U. MS/MS-based newborn and family screening detects asymptomatic patients with very-long-chain acyl-CoA dehydrogenase deficiency. J Pediatr 2003;143:335–342.
Arnold GL, Van Hove J, Freedenberg D, et al. A Delphi clinical practice protocol for the management of very long chain acyl-CoA dehydrogenase deficiency. Mol Genet Metab 2009;96:85–90.
Wilcken B. Medicine. Newborn screening: gaps in the evidence. Science 2013;342:197–198.
Hoffmann L, Haussmann U, Mueller M, Spiekerkoetter U. VLCAD enzyme activity determinations in newborns identified by screening: a valuable tool for risk assessment. J Inherit Metab Dis 2012;35:269–277.
Schiff M, Mohsen AW, Karunanidhi A, McCracken E, Yeasted R, Vockley J. Molecular and cellular pathology of very-long-chain acyl-CoA dehydrogenase deficiency. Mol Genet Metab 2013;109:21–27.
Andresen BS, Olpin S, Poorthuis BJ, et al. Clear correlation of genotype with disease phenotype in very-long-chain acyl-CoA dehydrogenase deficiency. Am J Hum Genet 1999;64:479–494.
Mathur A, Sims HF, Gopalakrishnan D, et al. Molecular heterogeneity in very-long-chain acyl-CoA dehydrogenase deficiency causing pediatric cardiomyopathy and sudden death. Circulation 1999;99:1337–1343.
Gregersen N, Andresen BS, Corydon MJ, et al. Mutation analysis in mitochondrial fatty acid oxidation defects: Exemplified by acyl-CoA dehydrogenase deficiencies, with special focus on genotype-phenotype relationship. Hum Mutat 2001;18:169–189.
Wanders RJA, Ruiter JPN, IJLst L, Waterham HR, Houten SM. The enzymology of mitochondrial fatty acid beta-oxidation and its application to follow-up analysis of positive neonatal screening results. J Inherit Metab Dis 2010;33:479–494.
Nouws J, Te Brinke H, Nijtmans LG, Houten SM. ACAD9, a complex I assembly factor with a moonlighting function in fatty acid oxidation deficiencies. Hum Mol Genet 2014;23:1311–1319.
Ventura FV, Costa CG, Struys EA, et al. Quantitative acylcarnitine profiling in fibroblasts using [U-13C] palmitic acid: an improved tool for the diagnosis of fatty acid oxidation defects. Clin Chim Acta 1999;281:1–17.
Manning NJ, Olpin SE, Pollitt RJ, Webley J. A comparison of [9,10-3H]palmitic and [9,10-3H]myristic acids for the detection of defects of fatty acid oxidation in intact cultured fibroblasts. J Inherit Metab Dis 1990;13:58–68.
Bastin J, Lopes-Costa A, Djouadi F. Exposure to resveratrol triggers pharmacological correction of fatty acid utilization in human fatty acid oxidation-deficient fibroblasts. Hum Mol Genet 2011;20:2048–2057.
Yamaguchi S, Li H, Purevsuren J, et al. Bezafibrate can be a new treatment option for mitochondrial fatty acid oxidation disorders: Evaluation by in vitro probe acylcarnitine assay. Mol Genet Metab 2012;107:87–91.
Olpin SE, Manning NJ, Pollitt RJ, Clarke S. Improved detection of long-chain fatty acid oxidation defects in intact cells using [9,10-3H]oleic acid. J Inherit Metab Dis 1997;20:415–419.
Vianey-Saban C, Divry P, Brivet M, et al. Mitochondrial very-long-chain acyl-coenzyme A dehydrogenase deficiency: clinical characteristics and diagnostic considerations in 30 patients. Clin Chim Acta 1998;269:43–62.
Baruteau J, Sachs P, Broué P, et al. Clinical and biological features at diagnosis in mitochondrial fatty acid beta-oxidation defects: a French pediatric study from 187 patients. Complementary data. J Inherit Metab Dis 2014;37:137–139.
Olpin SE, Manning NJ, Pollitt RJ, Bonham JR, Downing M, Clark S. The use of [9,10-3H]myristate, [9,10-3H]palmitate and [9,10-3H]oleate for the detection and diagnosis of medium and long-chain fatty acid oxidation disorders in intact cultured fibroblasts. Adv Exp Med Biol 1999;466:321–325.
Wanders RJ, IJlst L, Poggi F, et al. Human trifunctional protein deficiency: a new disorder of mitochondrial fatty acid beta-oxidation. Biochem Biophys Res Commun 1992;188:1139–1145.
Chegary M, Brinke Ht, Ruiter JP, et al. Mitochondrial long chain fatty acid beta-oxidation in man and mouse. Biochim Biophys Acta 2009;1791:806–815.
Merritt JL 2nd, Vedal S, Abdenur JE, et al. Infants suspected to have very-long chain acyl-CoA dehydrogenase deficiency from newborn screening. Mol Genet Metab 2014;111:484–492.
Bonnefont JP, Taroni F, Cavadini P, et al. Molecular analysis of carnitine palmitoyltransferase II deficiency with hepatocardiomuscular expression. Am J Hum Genet 1996;58:971–978.
Chegary M, Te Brinke H, Doolaard M, et al. Characterization of L-aminocarnitine, an inhibitor of fatty acid oxidation. Mol Genet Metab 2008;93:403–410.
Violante S, Ijlst L, van Lenthe H, de Almeida IT, Wanders RJ, Ventura FV. Carnitine palmitoyltransferase 2: New insights on the substrate specificity and implications for acylcarnitine profiling. Biochim Biophys Acta 2010;1802:728–732.
Berendse K, Ebberink MS, Ijlst L, Poll-The BT, Wanders RJ, Waterham HR. Arginine improves peroxisome functioning in cells from patients with a mild peroxisome biogenesis disorder. Orphanet J Rare Dis 2013;8:138.
Acknowledgements
E.F.D. is supported by a ZonMw grant (200320006) and Metakids (http:///www.metakids.nl). R.H.H. is supported by a VENI grant from ZonMw (91613050).
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Figure S1
(JPEG 725 kb)
Supplementary Figure S2
(JPEG 2182 kb)
Supplementary Table S1
(DOC 37 kb)
Supplementary Table S2
(DOC 65 kb)
Rights and permissions
About this article
Cite this article
Diekman, E., Ferdinandusse, S., van der Pol, L. et al. Fatty acid oxidation flux predicts the clinical severity of VLCAD deficiency. Genet Med 17, 989–994 (2015). https://doi.org/10.1038/gim.2015.22
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2015.22
Keywords
This article is cited by
-
Severity estimation of very-long-chain acyl-CoA dehydrogenase deficiency via 13C-fatty acid loading test
Pediatric Research (2022)
-
Management and diagnosis of mitochondrial fatty acid oxidation disorders: focus on very-long-chain acyl-CoA dehydrogenase deficiency
Journal of Human Genetics (2019)
-
Profiling of intracellular metabolites produced from galactose and its potential for galactosemia research
Orphanet Journal of Rare Diseases (2018)
-
Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle
Reviews in Endocrine and Metabolic Disorders (2018)
-
Residual N‐acetyl‐α‐glucosaminidase activity in fibroblasts correlates with disease severity in patients with mucopolysaccharidosis type IIIB
Journal of Inherited Metabolic Disease (2016)