Abstract
Purpose: To develop a model of offering population carrier screening for fragile X syndrome to nonpregnant women in primary care, using a program evaluation framework.
Methods: A three-phase approach included: (I) needs assessment exploring staff and client attitudes, and informing development of educational materials, questionnaires and protocols; (II) offering screening to women, with questionnaires at baseline (Q1) and another (Q2) 1-month later; (III) genetic counseling for test-positive women and interviews with a subgroup of participants.
Results: Of 338 volunteering for Phase II, 94% completed Q1, 59% completed Q2, and 20% (N = 65) chose testing revealing one premutation carrier and three gray zone results; 31 women were interviewed. Tested women had more positive attitudes toward screening (Q1: P < 0.001; Q2: P < 0.001) compared with untested, although there was no significant difference in mean knowledge scores or anxiety. Women generally supported being offered prepregnancy screening; however, reasons against being tested included: not currently planning a family; perceiving benefits of screening as unimportant; and having to return for testing.
Conclusion: This is the first prospective study exploring informed decision-making for fragile X syndrome carrier screening, using a thorough process of consultation, with no apparent harms identified. It provides a model for development of future genetic screening programs.
Similar content being viewed by others
Main
Population-based genetic screening programs can be implemented for different purposes.1 Often this screening occurs during pregnancy with restricted options for the couple; however, screening before pregnancy can provide a more appropriate timeframe for reproductive decision-making and broadens options (e.g., adoption, gamete donation, preimplantation genetic diagnosis, choosing not to have a family) according to values and beliefs.
Criteria exist for offering population-based genetic screening programs.1,2 It is essential that studies are undertaken to assess how such testing can best be offered, at what age screening should be done, how information is best delivered in a clear and nondirective manner to ensure informed decision-making, and how results should be followed up. These considerations are now recognized to be just as critical as traditional epidemiological analyses and cost-benefit evidence for policy development related to screening program implementation.1,3,4 Ideally, studies addressing acceptability of population genetic screening should assess attitudes to screening and experiences of participating in the screening program, including impact of receiving test results. Studies should explore these issues before implementing the program (i.e., needs assessment) to inform development of the program, as uptake of testing alone should not be considered the sole measure of acceptability.
Carrier screening for fragile X syndrome (FXS), unlike many other inherited conditions, can serve a dual purpose: as well as identifying individuals at reproductive risk, such screening also has implications for the personal health of the individual identified as a carrier. FXS, an X-linked condition, is the most common cause of inherited intellectual disability and is found in approximately 1 in 4000 men and in 1 in 4000–8000 women.5 It is second only to Down syndrome as the leading genetic cause of intellectual disability. Other features with variable severity include some physical and medical characteristics and serious behavioral and emotional problems,6 with women generally having a less severe phenotype than men. Although FXS is not curable, interventions can reduce the medical and behavioral symptoms, and currently there are promising experimental data suggesting new pharmacotherapies that have the potential to treat some of the brain pathologies.7–10 FXS is usually caused by an increase in the number of trinucleotide (CGG) repeats in the promoter of the FMR1 gene. This repeat length varies in the population, with 6–44 repeats considered to be the “normal” range, whereas people who are affected have a hypermethylated repeat length >200 (full mutation). A repeat length of 55–200 is called the premutation, and in this study we refer to this as the carrier state. Carriers may have some mild learning or emotional difficulties, and a risk of developing a late-onset neurodegenerative condition with tremor/ataxia and a 20% risk of developing premature ovarian failure in women. Adding to the complexity, an allele of intermediate length (“gray zone”, 45–54 repeats) may increase to a premutation length allele when transmitted to offspring. A more current view is emerging that repeat length can be considered as a continuum ranging from small to a large number of repeats, associated with a concomitant phenotype spectrum.11 The repeat length is unstable over a certain size and can expand when passed onto offspring through female carriers,12–14 thus it is female carriers who are at greatest risk of having affected children as well has having personal health risks. Frequency of carrier females has been suggested as 1 in 259,15 with a more recent estimate of 1 in 157.16
FXS is usually diagnosed by case-finding with carriers identified through cascade testing of family members, yet the majority of carriers remain undetected.5,17 There have been discussions regarding the feasibility, cost-effectiveness, and cost-benefit of population-based carrier screening for FXS, with arguments generally in favor.18–23 Others have suggested that the decision to screen should focus on medical, social, psychological, and ethical considerations.21 Guidelines from the American College of Medical Geneticists state that population carrier screening for FXS is not recommended except within well-defined clinical research protocols,24 because of the difficulties around counseling and education regarding the meaning and interpretation of results.25 Nevertheless, such screening does occur in clinical practice in a number of centers, predominantly during pregnancy, especially in Israel16 and parts of the United States.26 A few studies have examined the attitudes of participants toward being screened, either within a testing protocol27–29 or in the absence of offering testing.30 However, there have been little published data describing decision-making and psychosocial consequences of such screening in women with no family history of FXS. Only in one small study of 20 women was there an attempt to evaluate whether participants made an informed decision to be tested.28 Furthermore, no studies have reported conducting a needs assessment with the target population to inform the development of the screening program or applying a model of program evaluation.31,32 Adequate education and counseling are critical to any screening program to ensure that there is informed decision-making; that test choice is respected; and that psychosocial consequences are minimized. This is especially relevant for conditions such as FXS, with its complexity of inheritance and personal and reproductive sequelae.25,30
The purpose of this study was to pilot a model for developing and evaluating population-based genetic screening programs. We describe here a three-phase pilot study of carrier screening for FXS in a population of women in a primary care setting. The focus was on prepregnancy in consideration that this would afford women the greatest number of reproductive options, especially given the relatively higher prevalence of premature ovarian failure in carriers.
SUBJECTS AND METHODS
Ethics
Ethics approval for all phases was obtained from the Human Research Ethics Committee of Family Planning Victoria (FPV) and each participant provided written informed consent.
The primary care setting
This study was conducted at FPV. This organization is publicly funded with a small annual fee for clients, waived for low-income earners. Staff includes physicians, nurses, educators, and administrators. FPV clients receive advice and medical attention for contraception, pregnancy, other sexually related and women's health medical information, and can attend via an appointment clinic or a “drop-in” clinic.
Phase I
A needs assessment was conducted with staff and clients of FPV to ascertain their views, understanding, interest and concerns regarding the possible introduction of carrier screening for FXS for nonpregnant women. This information was used to develop protocols and information resources for offering the screening program. An interview was held with the CEO and separate focus groups were conducted with staff and clients, preceded by a short presentation about FXS. Clients were given a small monetary reimbursement for their time. Sampling continued until data reached saturation i.e., no further themes emerged from the discussions.
The data from these discussions were used to inform development of a brochure and two questionnaires for Phase II. Design and content of the brochure was initially developed by staff at Genetic Health Services Victoria and Murdoch Childrens Research Institute with expertise in clinical genetics, genetics education, genetic counseling and public health genetics, and by members of the Fragile X Alliance Inc., a joint clinical and patient support organization. The brochure was then sent to participants in the needs assessment and their opinions solicited over the telephone, resulting in successive rounds of revisions. Questionnaires were designed to examine informed choice, rationale for choosing testing, and indicators of psychological sequelae. Thus, questionnaire 1 (Q1) included the following items: (1) awareness of FXS; (2) knowledge of FXS; (3) attitudes toward carrier screening in general; (4) attitudes toward carrier screening for FXS (attitude scale modified from Ref. 33); (5) the state component of the short form of the Spielberger State-Trait Anxiety Inventory;34,35 (6) decision to undergo testing; (7) reasons for and against testing (closed items plus comments); (8) sociodemographic details; and (9) opportunity to comment on the information brochure. Questionnaire 2 (Q2) included items that asked about women's experience of being in the screening program and items 2, 4, 5, 6, and open-ended 7 from Q1. The questionnaires were reviewed by the genetics experts listed above using a modified Delphi consultation to achieve consensus.36 Finally, a forum was held with staff of FPV to discuss recruitment and testing protocols.
Phase II
Women, aged 18 or older, not pregnant at the time of recruitment and who could read, write, and speak English, were recruited into the study. Participation in the study required that they complete Q1 at the time of recruitment, be offered carrier testing for FXS and then complete Q2 1 month later, or after receiving their result if they chose to be tested.
The protocols for recruitment differed somewhat according to which clinic the woman attended. The main differences were that women who made an appointment were sent the brochure and participant information statement before attending, were recruited by an FPV nurse (who has been specifically trained regarding FXS carrier screening), and could be tested at the time of recruitment. Women attending the drop-in clinic received the written materials in the waiting room and were recruited by a research genetic counselor. There was a human research ethics committee requirement that women attending the drop-in clinic return at a later date to give a sample for testing if they chose to be tested, as these women only received their information on the day of recruitment. All women had the opportunity to discuss the study and issues around carrier testing for FXS with the research genetic counselor or nurse at the time of recruitment and at later stages should they wish. Tested women received a normal result by mail, or were telephoned if they were identified as having a gray zone or carrier result and invited to attend for genetic counseling at Genetic Health Services Victoria.
Phase III
A sample of women completing both questionnaires who further consented to be interviewed were recontacted after completing Q2, or after receiving genetic counseling, and invited for follow-up interviews to discuss their experiences of participating in the screening program in greater depth. Participants also received a small monetary reimbursement for their time. Sampling of women continued until the emerging data reached saturation.37
Qualitative data analysis
Focus groups and interviews were audiotaped, transcribed, and imported into NVivo (QSR International Pty Ltd, Melbourne, Australia), to organize data and facilitate coding. Transcripts were coded and analyzed for themes using a constant comparison approach.38 Transcripts from Phase I and Phase III were analyzed separately and coding was carried out by at least two independent researchers to provide rigor of analysis. Pseudonyms were used for Phase III participants. Open ended responses from the questionnaires were also coded: using thematic analysis in Q1; and content analysis in Q2, using codes based on themes arising from Q1, thereby allowing these categories to be quantified.
Quantitative data analysis
Data were analyzed using SPSS version 15.01 (Statistical Program for the Social Sciences, SPSS Inc, Chicago, IL). Descriptive statistics were used to describe the sociodemographic, psychological, and practical characteristics of the participants. t Tests were used to determine the statistical significance of the differences in mean scores between groups. Paired t tests were used to assess the significance in mean differences over time (between Q1 and Q2). Chi-squared tests were used to assess the statistical significance of associations between demographic characteristics and test uptake. The McNemar test for related samples was used to assess differences in proportions over time within tested and untested groups. A p-value <0.05 was considered statistically significant.
Genetic testing of fragile X syndrome
Testing on blood samples was provided free of charge to the participants. The protocol described below is used for diagnostic testing at Victorian Clinical Genetics Services Pathology, Melbourne, Australia.
FAM™ labeled primers A (5′-GGAACAGCGTTGATCACGTGACGTGGTTTC-3′) and 571R (5′-GGGGCCTGCCCTAGAGCCAAGTACCTTGT-3′) were selected to span the CGG triplet repeat region in the 5′ untranslated region of exon 1 within FMR1. Amplifications were performed in a 25 μL reaction volume containing 2.5 μL dNTPs (2 mM), 0.5 μL Pfu (Stratagene) exo(-)enzyme (2.5 U/μL), 2.5 μL Pfu buffer, 3.1 μL DMSO, 0.5 μL primer A (165 ng/μL), 0.5 μL primer 571R (165 ng/μL) and 45–60 ng genomic DNA. Thermocycling program (Gene Amp@ PCR System 9700): 5 minutes denaturation at 98°C; 35 cycles at 98°C for 1 minute, 62°C for 1 minute, 72°C for 2 minutes; final extension of 5 minutes at 72°C. Alleles were sized by capillary electrophoresis using an automatic sequencer (MegaBACE™ 1000 – GE HealthCareAmersham) with an error of measurement of ± two repeats. Southern blot analysis was performed on samples which demonstrated a single allele on PCR analysis, typically 40% of samples with this procedure. PstI digested DNA was hybridized with probe pfxa3.12
RESULTS
Phase I
Two focus groups were held with staff (N = 12) and three with clients (N = 18) of FPV (Table 1). A number of themes emerged relating to appropriateness of offering carrier screening for FXS to women in general and more specifically at FPV in the future. Overall, participants believed that carrier screening for FXS should be offered to all women in the general population (one staff member disagreed with this), although there was some discussion about ethical issues and concerns about “where we draw the line” as a society regarding which conditions should be tested. There were many comments that awareness about FXS should be increased in the community and that informed consent and education about FXS are vital if screening is to take place (Table 2; quotes 1 to 3).
Both staff and clients believed that the test should be available to all women, irrespective of their socioeconomic status, i.e., cost should not be a barrier. Strong perceived benefits of screening were that it increased women's reproductive options, especially if offered before pregnancy; and that it allowed women to prepare for the birth of a child with an intellectual disability, in women who choose to continue a pregnancy with an affected child. Relief for women found to have a normal test result was also mentioned, although anxiety as a potential harm emerged as a strong theme in both groups. When asked when carrier screening for FXS should be offered there were mixed views: staff most commonly supported screening during the early stages of pregnancy whereas, generally, clients said it should be offered without specific consideration of pregnancy (Table 2; quotes 4 and 5). Most participants were in favor of offering screening at FPV provided that staff was suitably trained and that genetic counseling and/or referral to other supports/services was available for those women found to be carriers.
Phase I informed development of the brochure content and layout, with repeated modifications following feedback by genetics experts, client, and staff participants (N = 11) from Phase I. See Table 3 and Figure 1 for description of content of the final brochure. Findings from Phase I also guided development of the questionnaires with consensus achieved after two rounds of modifications based on the Delphi consultation with 10 experts. After a forum with all staff at FPV, two slightly different recruitment methods were chosen to reflect operation logistics of the clinic. Eight FPV nurses received training about FXS and one nurse was selected to manage the appointment clinic, recruiting and pretest counseling, with the support of the research genetic counselor.
Phase II
One hundred seventeen women who had made appointments were contacted about the study and deemed eligible to participate. Of these, 42 agreed to participate (36%), 47 declined (40%), and 28 (24%) cancelled or failed to attend the appointment. In the drop-in clinic, 309 women were approached in the waiting room and 285 (92%) agreed to participate. Thus, the combined participation rate from the two clinics was 77%. Also 11 staff members asked to enter the study and were recruited in the same way as clients. Although we did not specifically document how much time was spent discussing the study with each woman, our impression is that on average this took around 15–20 minutes. For a small number of women, especially those who were uncertain about whether to be tested, there were multiple contacts with the research genetic counselor, anytime up to 2 hours in total.
Of the final 338 women who were recruited, 318 completed Q1 (94%) and 187 completed Q2 (59%). Of those who completed Q1, 65 women chose to be tested (20% uptake): 27 (64%) from appointment clinic, 27 (10%) from drop-in clinic and 11 staff (100%). One woman received a carrier (premutation) result and three received a gray zone result. Participants' sociodemographic characteristics are shown in Table 1. Seventy-seven percent of tested women had a partner compared with 57% of untested women (P = 0.004). Tested women were also significantly older (tested mean age 33.3 years, untested 28.8 years, P < 0.001). However, there was no significant difference between tested and untested women in terms of whether or not they had children (35% of tested vs. 25% of untested women, P = 0.1) or had completed tertiary education (23% tested vs. 34% untested, P = 0.1). Most women had no family history of FXS and a family history was not associated with testing (8% of tested vs. 15% of nontested, P = 0.1).
Knowledge questions were generally answered well with around two-thirds of women answering seven or more correctly at each time-point (Table 4). There was no significant difference between mean knowledge scores of tested compared with untested women in Q1 (6.6 vs. 6.8, P = 0.5) and in Q2 (7.2 vs. 6.8, P = 0.2). Neither the tested nor untested groups showed a significant change in mean knowledge score over time (tested: Δ = 0.52, P = 0.08; untested: Δ = 0.27, P = 0.12).
Women were overwhelmingly in support of FXS carrier screening being available to the general community, although less positive about genetic testing more generally (Table 4). However, the proportion of women with a positive attitude toward FXS carrier testing for themselves was significantly higher for tested than untested women in both Q1 (P < 0.001) and Q2 (P < 0.001). Over time, the tested women showed no significant change in the proportion with a positive attitude (from 77–84%, P = 0.5) whereas the proportion of untested women with a positive attitude decreased (from 46–24%, P = 0.001).
Slight differences in the mean anxiety scores (Table 4) of tested compared with untested women were not statistically significant in Q1 (P = 0.5) or Q2 (P = 0.1). There was a reduction in mean anxiety score over time for tested women (Δ = −3.6, P = 0.02) and no significant change for the untested group (Δ = 0.8, P = 0.5).
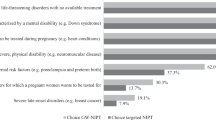
In Q1 women were asked about their intention to have the test and to tick the reasons that were important in making their decision. One hundred sixty-six women (52%) said they wanted to have the test and 71 (22%) were unsure, although ultimately only 65 (20%) actually were tested. The majority of women who wanted to be tested said they would do so because they wanted to know if they were a carrier (90%) and wanted to know about their own health and the chance of having a child with FXS (Fig. 2A). This figure shows these data categorized into those women who actually were tested (dark bars) compared with those who intended to, but who subsequently were not tested (light bars). In Q1, 79 women (25%) said they would not have the test and the two main reasons they gave were that they did not think it was relevant to them and that they were not currently planning a family (Fig. 2B).
Reasons for and against choosing to have the carrier test for fragile X syndrome. Question (in Q1) was asked regarding whether the woman was going to have the test. A, If the response was “yes”, then the various reasons that were important in making the decision to be tested were listed. B, If response was “no” then the various reasons that were important in making the decision not to be tested were listed. Women could tick as many as applied to them, and the percentage of women ticking a particular response is indicated. Note: The dark bars in (A) refer to the women who actually did get tested compared with the lighter bars that refer to women who intended to be tested but who did not ultimately have the test for various reasons.
In Q2 women were asked whether they were actually tested or not and to give the main reason for their decision, this time by providing open-ended comments, which were generally fairly brief. Of the 45 women who had intended to be tested but who did not do so, 60% mentioned lack of time and the inconvenience of having to return to give the blood sample, with 19% remarking that they were not planning or had completed a family, whereas other comments included not wanting to know and not believing they were at risk of being a carrier. Of the 71 women who had indicated they were unsure about being tested, 4 (5%) were tested, 26 (37%) did not complete Q2 and 41 (58%) were not tested. These women gave a similar range of reasons for not returning to have the test, including lack of time or because they were not planning a family. However, their most common reason was because they would prefer not to know, either because it would not change their mind about having children or because they would be worried about knowing. No women who were tested regretted being tested whereas nine women who were not tested regretted their decision.
Phase III
Of the 318 women in the study, 233 (73%) consented to being invited for a follow-up interview. After completion of Q2, interviews were requested from a selected sample of 53 women, representing a range who had been tested, those who had intended to be tested but were not and women who were tested, including three test-positive women (one woman with a gray zone result had not consented to be contacted). Interviews were ultimately conducted with 31 women (of the remaining 22 women, 13 (25%) declined to participate in the interviews whereas others (17%) could not be contacted further): 18 nontested women (including 10 who initially intended to have the test); 13 tested women, including the woman who received the carrier result and 2 of the women with gray zone results. As analysis of the transcripts of these interviews progressed it was apparent that no new themes were emerging and so no further participants were contacted for interviews. The demographic characteristics of the women interviewed were representative of the overall participants in Phase II (Table 1).
Overall women were supportive of preconception carrier testing (Table 5; quote 1) and discussed a number of issues, to be reported elsewhere, but here we briefly describe the main findings. The main factors that influenced test choice included: life stage of the woman, i.e., if she was thinking about having children she was more likely to consider having the test (Table 5; quotes 2 and 3); whether the woman had any experience with health problems, i.e., if so she was more likely to consider having the test (Table 5; quote 4); and the woman's perception of the benefits of carrier testing (Table 5; quotes 5 and 6). The tested women generally did not express regret (Table 5; quote 7), as shown in Phase II data, although one woman with a gray zone result was ambivalent as she was uncertain about what to do with the information in terms of discussing with family members (Table 5; quotes 8 and 9). The woman with the carrier result was very positive about her decision to be tested (Table 5; quote 10), and subsequently other family members also had genetic counseling and genetic testing.
The majority of women who commented on the brochure (in both Phase II and Phase III) made favorable remarks, and were especially positive about the way in which the gene length was depicted (Fig. 1; Table 5; quote 11). Women who were interviewed, however, felt that the symptoms of FXS needed further explanation regarding the full range of severity (Table 5; quote 12).
DISCUSSION
Population-based carrier screening has been offered to women with no family history of FXS, mostly in prenatal settings,22,26,27,39,40 with preconception screening to subgroups of women only reported in three studies,22,30,40 even though families with experience of FXS generally consider that this is the best time to offer testing.41–43 A few studies have ascertained the attitudes of participants toward being screened,27,28,30 with one small prospective study also looking at knowledge.28 This is the first study reporting use of a program evaluation approach to develop a carrier screening program for FXS, i.e., a needs assessment with stakeholders to inform the development of the program, implementation, and evaluation that includes assessing informed choice and psychosocial consequences, using both quantitative and qualitative data collection methods. Overall, the results suggest that women who were offered carrier screening made a decision about testing that was based on sufficient knowledge and was consistent with their values.44 Thus there were relatively high knowledge levels in those tested and not tested, and women who chose testing had more positive attitudes to being tested for FXS carrier status, as one might expect. Furthermore, in this study women had time, and thus the opportunity, to consider the pros and cons of being tested. In general, being offered screening, and accepting testing, did not increase anxiety, although we cannot draw any specific conclusions about the women who received a positive result due to the small numbers (one premutation and three gray zone results).
Although this article describes a model for developing such a screening program, this is a small pilot study and we acknowledge some limitations with the methodology. The quoted statistical significance levels were for each characteristic considered independently and should be interpreted conservatively because of multiple statistical testing. Other limitations were often related to the logistics of the clinical setting. Thus, the feasibility of offering screening at FPV varied depending on the type of clinic. It was apparent that women who received information before attending the appointment were less likely to participate in the study than women who were recruited at the drop-in clinic while waiting to see the doctor (36% vs. 92%). However, we cannot infer whether this was because women in the appointment group did not want screening or simply did not want to enter the study. On the other hand, there was greater uptake of testing in women attending the appointment clinic compared with the drop-in clinic (64% vs. 10%), although 52% of women in the drop-in clinic had indicated their intention to be tested. A noteworthy barrier to uptake of testing for women at the drop-in clinic was the requirement that they return to provide a blood sample. Lack of time and this inconvenience was cited by 60% of women who initially had intended to be tested but who subsequently were not. Nevertheless, this requirement did provide women with the opportunity to fully consider the implications of being tested, and follow-up interviews indicated that generally women who did not return to have the test because of lack of time were also uncertain about the test for other reasons. It is interesting to note that in women who had been unsure whether to be tested or not, one of their main reasons for not returning for testing was that they felt they did not want to know as this would make them worry and, even if found to be a carrier, would not change their mind about having children.
This pilot study was conducted in only one particular health care setting, with an apparently well-educated group of women participating in our study (∼68% with tertiary level education), and it could be argued that this is not a generalizable study population. The Australian Bureau of Statistics 2006 census data (http://www.abs.gov.au/) reveal that 73% of women living in the geographical region serviced by this clinic and in the age range 25–34 years (i.e., encompassing the mean ages for the women in our study) have a tertiary level education, whereas in the overall Australian women population of this age range this proportion is 55%. Therefore, while the women in our study are indeed well-educated, they are not too dissimilar compared with the general population. Eligibility to participate was limited to women who could speak, read, and write English, although we are aware that for some of the participants English was not their first spoken language. As this was a pilot study, questionnaires, educational, and study materials were produced only in English, due to cost and convenience. In the needs assessment (Phase I), however, the demographic characteristics of the women were comparable with those of women in Phases II and III. Thus, if a carrier screening program were expanded to include women who have poor English language skills, it would be necessary to ensure that a needs assessment also includes such women, and that this would inform production of appropriate materials.
The selection of items to be included in the questionnaires reflected the aims of assessing women's knowledge, attitudes and reasons for choosing testing (all indicators of informed choice) and anxiety as a psychological outcome. Furthermore, the interviews provided in-depth exploration of women's experiences of participating in the study and included tested and nontested women. The qualitative data support and extend the data collected in the questionnaires. However, we did not attempt to quantify informed choice for each individual woman. Although there is an instrument that constructs a measure of informed choice,33,45 this has only been validated for prenatal screening for Down syndrome, and does not include deliberation (weighing up of pros and cons with consideration of risks and consequences). Since our study commenced, a new instrument for informed decision-making has been published that includes a deliberation scale, although again it has only been validated for Down syndrome screening in pregnancy.44 Nevertheless, such a tool would be useful to include in further studies of carrier screening, substituting knowledge questions about FXS instead of congenital defects.
In developing our model, we decided to focus on nonpregnant women for our pilot study. Preconception carrier screening for FXS has been considered to be difficult in practice. Murray et al.46 have stated that: “In the absence of pilot studies it is difficult to judge how feasible and acceptable preconception screening for FXS would be,” a view echoed by Sherman who advocates the need for more studies in this area.5 Anido et al.30 have suggested that women in the general population who do not have a family history of FXS would have difficulties considering the implications of a genetic carrier test and that they would not be prepared for the consequences of a positive result. These conclusions were based on their study in which women who had been recruited through another research study were offered carrier screening, with a sample of test-negative women participating in focus groups,30 and interviews with eight women found to be carriers.29 However, in our pilot study, there was strong support overall for offering FXS carrier screening before pregnancy to women in the general population, and it would appear that the majority of women in our study made an informed decision about whether to be tested themselves. The woman who received a carrier result had no regrets about being tested and felt empowered; indeed this led to cascade testing of other family members. Only 10% of decliners said in Q1 that they did not want to know at all. For nontested women, their awareness of FXS has now been raised, so that they may consider testing in the future, e.g., at a time closer to pregnancy.
Another point to consider for future screening programs is that a number of the women who declined screening believed that it was not relevant to them because of a lack of family history; thus, it is obviously important to emphasize that a family history is not necessary to be at risk. Indeed, the women who were found to have a premutation or gray zone result had no previously documented family history of FXS.
Our uptake of 20% is similar to other US reports (21%39 and 7.9%26), although less than in two Israeli reports (80%40 and 79%22) and a Finnish study (85%27). These programs predominantly tested pregnant women with prevalence data as the main outcomes, whereas attitudes to testing were not assessed, except for a small subgroup retrospectively at postscreening in the Finnish study, so it is difficult to determine to what extent women had made an informed decision to be tested. We are currently planning a larger study in preconception primary care with modifications to the assay,47 sampling protocol and the brochure to improve access for women to have carrier screening, without compromising time for deliberation and informed decision-making.
In our study, we did not attempt to make any economic assessment as this was a pilot study with relatively small numbers, although in future larger studies this would be an important component in terms of assessing feasibility. The test was offered at no charge to the women (research funds supported the cost of the test for the laboratory) as this was a clear message from the needs assessment. Interestingly, although 44% of women ticked the “test at no charge” box in Q1 as one of the reasons for wanting to be tested, only one woman was who tested gave that as the main reason in the open-ended comments in Q2. Australia has a socialized health care system, in which public and private models operate in parallel. During pregnancy, a woman may have a screening test (second trimester maternal serum screening and ultrasound) that is publicly funded and give birth in a public hospital, with no/minimal out of pocket expenses. Alternatively, other screening tests (e.g., first combined maternal serum screening) are offered through the private system and a woman may choose to give birth in a private hospital, partly funded by private health insurance with usually some out of pocket expenses. Thus, for some women paying for a screening test will be acceptable whereas not for others. Again, further exploration of this would be important in future studies, impacting on any economic assessment and informing health care policy, and would depend to a certain extent on the clinical setting.
In our study, we elected to inform women who were found to have a gray zone result. Counseling about the implications of gray zone result is a challenging area of carrier screening for FXS. In our brochure, we referred to a person having a gray zone result as having an intermediate-length gene that can increase to a medium-length gene (carrier/premutation) when passed on from mother to child, and that women with such a result may have children who are carriers and, therefore, grandchildren who may be affected by FXS. This information was repeated in the genetic counseling session with the three women who had a gray zone result. There is much ethical debate about how much information should be given after genetic testing when the outcome is uncertain. However, we considered it unethical to withhold such information from women, rather preferring to provide the information so that they could decide for themselves.
Gray zone allele frequency in women has been reported as 1 in 5248 and 1 in 69,22 whereas we found three in 65 women (∼1 in 22) and one carrier. We cannot, however, draw any conclusions about allele frequencies from our study as the numbers were small and this was not the purpose of the research. As research continues to expand our understanding of the role of FMR1, it is becoming apparent that the phenotype is a spectrum associated with the repeat size continuum, and very recent data suggest that the gray zone allele may also be linked to premature ovarian failure49,50 and possibly other toxic gain of functions.51 Although the clinical implications are complex and often uncertain, this alone is not a reason to withhold information, as managing uncertainty is a common theme in genetic counseling. For future studies, it will be important to give due consideration to our changing understanding of clinical phenotype and genotype correlations, with a focus on educational and psychosocial outcomes.
In summary, this is the first prospective pilot study of carrier screening for FXS that adopts a program evaluation framework and provides data on women's knowledge, attitudes, and decision-making. This study reinforces women's preference for preconception carrier screening. Furthermore, the process used in its development included extensive consultation and feedback from the target population and their health care providers. This process is presented as a model for future genetic screening programs where testing for susceptibility to complex disorders, pharmacogenomics, and nutrigenomics will pose comparable challenges for informed decision-making and should include rigorous development and evaluation before, during and after their implementation.
References
Godard B, ten Kate L, Evers-Kiebooms G, Ayme S . Population genetic screening programmes: principles, techniques, practices, and policies. Eur J Hum Genet 2003; 11 ( suppl 2): S49–87.
Wilson JM, Jungner G . Principles and practice of screening for disease. Geneva: WHO, 1968.
Burke W, Coughlin SS, Lee NC, Weed DL, Khoury MJ . Application of population screening principles to genetic screening for adult-onset conditions. Genet Test 2001; 5: 201–211.
Khoury MJ, McCabe LL, McCabe ER . Population screening in the age of genomic medicine. N Engl J Med 2003; 348: 50–58.
Sherman S Epidemiology. In: Hagerman RJ, Hagerman PJ, editor. Fragile X syndrome: diagnosis, treatment, and research. Baltimore: Johns Hopkins University Press, 2002; 136–168
Hagerman RJ, Hagerman PJ . Fragile X syndrome: diagnosis, treatment, and research. Baltimore: Johns Hopkins University Press, 2002.
Tucker B, Richards RI, Lardelli M . Contribution of mGluR and Fmr1 functional pathways to neurite morphogenesis, craniofacial development and fragile X syndrome. Hum Mol Genet 2006; 15: 3446–3458.
Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP . Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 2005; 49: 1053–1066.
Nakamoto M, Nalavadi V, Epstein MP, et al. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc Natl Acad Sci USA 2007; 104: 15537–15542.
McBride SM, Choi CH, Wang Y, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 2005; 45: 753–764.
Hagerman PJ, Hagerman RJ . The fragile-X premutation: a maturing perspective. Am J Hum Genet 2004; 74: 805–816.
Fu YH, Kuhl DP, Pizzuti A, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 1991; 67: 1047–1058.
Nolin SL, Lewis FA III, Ye LL, et al. Familial transmission of the FMR1 CGG repeat. Am J Hum Genet 1996; 59: 1252–126.
Snow K, Doud LK, Hagerman R, Pergolizzi RG, Erster SH, Thibodeau SN . Analysis of a CGG sequence at the FMR-1 locus in fragile X families and in the general population. Am J Hum Genet 1993; 53: 1217–1228.
Rousseau F, Rouillard P, Morel ML, Khandjian EW, Morgan K . Prevalence of carriers of premutation-size alleles of the FMRI gene—and implications for the population genetics of the fragile X syndrome. Am J Hum Genet 1995; 57: 1006–1018.
Berkenstadt M, Ries-Levavi L, Cuckle H, Peleg L, Barkai G . Preconceptional and prenatal screening for fragile X syndrome: experience with 40,000 tests. Prenat Diagn 2007; 27: 991–994.
Wildhagen MF, van Os TA, Polder JJ, ten Kate LP, Habbema JD . Efficacy of cascade testing for fragile X syndrome. J Med Screen 1999; 6: 70–76.
Palomaki GE . Population based prenatal screening for the fragile X syndrome. J Med Screen 1994; 1: 65–72.
Finucane B . Should all pregnant women be offered carrier testing for fragile X syndrome?. Clin Obstet Gynecol 1996; 39: 772–782.
Meadows KL, Sherman SL . Fragile X syndrome: examination of issues pertaining to population-based screening. Screening 1996; 4: 175–192.
Wildhagen MF, van Os TA, Polder JJ, ten Kate LP, Habbema JD . Explorative study of costs, effects and savings of screening for female fragile X premutation and full mutation carriers in the general population. Comm Genet 1998; 1: 36–47.
Toledano-Alhadef H, Basel-Vanagaite L, Magal N, et al. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet 2001; 69: 351–360.
Musci TJ, Caughey AB . Cost-effectiveness analysis of prenatal population-based fragile X carrier screening. Am J Obstet Gynecol 2005; 192: 1905–1912.
Sherman S, Pletcher BA, Driscoll DA . Fragile X syndrome: diagnostic and carrier testing. Genet Med 2005; 7: 584–587.
McConkie-Rosell A, Finucane B, Cronister A, Abrams L, Bennett RL, Pettersen BJ . Genetic counseling for fragile X syndrome: updated recommendations of the national society of genetic counselors. J Genet Couns 2005; 14: 249–270.
Cronister A, DiMaio M, Mahoney MJ, Donnenfeld AE, Hallam S . Fragile X syndrome carrier screening in the prenatal genetic counseling setting. Genet Med 2005; 7: 246–250.
Ryynanen M, Heinonen S, Makkonen M, Kajanoja E, Mannermaa A, Pertti K . Feasibility and acceptance of screening for fragile X mutations in low-risk pregnancies. Eur J Hum Genet 1999; 7: 212–216.
Fanos JH, Spangner KA, Musci TJ . Attitudes toward prenatal screening and testing for Fragile X. Genet Med 2006; 8: 129–133.
Anido A, Carlson LM, Sherman SL . Attitudes toward fragile X mutation carrier testing from women identified in a general population survey. J Genet Couns 2007; 16: 97–104.
Anido A, Carlson LM, Taft L, Sherman SL . Women's attitudes toward testing for fragile X carrier status: a qualitative analysis. J Genet Couns 2005; 14: 295–306.
Ovretveit J, Planning and managing an evaluation In: Ovretveit J, editor. Evaluating health interventions: an introduction to evaluation of health treatments, services, policies and organizational interventions. Buckingham and Philadelphia: Open University Press, 1998; 158–180
Metcalfe SA, Aitken MA, Gaff CL . The importance of program evaluation: how can it be applied to diverse education settings?. J Genet Couns 2008; 17: 170–179.
Michie S, Dormandy E, Marteau TM . The multi-dimensional measure of informed choice: a validation study. Patient Educ Couns 2002; 48: 87–91.
Spielberger C, Gorusch R, Luchene R . State trait anxiety inventory: manual. Palo Alto: Consulting Psychologists Press, 1983.
Marteau TM, Bekker H . The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psych 1992; 31: 301–306.
Hasson F, Keeney S, McKenna H . Research guidelines for the Delphi survey technique. J Adv Nursing 2000; 32: 1008–1015.
Strauss A, Corbin J . Basics of qualitative research: techniques and procedures for developing grounded theory. Thousand Oaks, CA: SAGE, 1998.
Glasser BG, Strauss AL . The discovery of grounded theory—strategies for qualitative research. New York: Aldine De Gruyter, 1967.
Spence WC, Black SH, Fallon L, et al. Molecular fragile X screening in normal populations. Am J Med Genet 1996; 64: 181–183.
Pesso R, Berkenstadt M, Cuckle H, et al. Screening for fragile X syndrome in women of reproductive age. Prenat Diagn 2000; 20: 611–614.
McConkie-Rosell A, Spiridigliozzi GA, Lafolla T, Tarleton J, Lachiewicz AM . Carrier testing in the fragile X syndrome: attitudes and opinions of obligate carriers. Am J Med Genet 1997; 68: 62–69.
McConkie-Rosell A, Spiridigliozzi GA, Rounds K, et al. Parental attitudes regarding carrier testing in children at risk for fragile X syndrome. Am J Med Genet 1999; 82: 206–211.
Skinner D, Sparkman KL, Bailey DB Jr Screening for Fragile X syndrome: parent attitudes and perspectives. Genet Med 2003; 5: 378–384.
van den Berg M, Timmermans DR, ten Kate LP, van Vugt JM, van der Wal G . Informed decision making in the context of prenatal screening. Patient Educ Couns 2006; 63: 110–117.
Marteau TM, Dormandy E, Michie S . A measure of informed choice. Health Expect 2001; 4: 99–108.
Murray J, Cuckle H, Taylor G, Hewison J . Screening for fragile X syndrome: information needs for health planners. J Med Screen 1997; 4: 60–94.
Tassone F, Pan R, Amiri K, Taylor AK, Hagerman PJ . A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn 2008; 10: 43–49.
Murray A, Youings S, Dennis N, et al. Population screening at the FRAXA and FRAXE loci: molecular analyses of boys with learning difficulties and their mothers. Hum Mol Genet 1996; 5: 727–735.
Bodega B, Bione S, Dalpra L, et al. Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum Reprod 2006; 21: 952–957.
Bretherick KL, Fluker MR, Robinson WP . FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet 2005; 117: 376–382.
Loesch DZ, Bui QM, Huggins RM, Mitchell RJ, Hagerman RJ, Tassone F . Transcript levels of the intermediate size or grey zone fragile X mental retardation 1 alleles are raised, and correlate with the number of CGG repeats. J Med Genet 2007; 44: 200–204.
Acknowledgements
This work was supported by Fragile X Alliance Inc, Murdoch Childrens Research Institute and The University of Melbourne. Alison Archibald is supported by an Australian Postgraduate Award Scholarship.
We thank Lynn Jordan and Dr Hennie Williams for their support in allowing us to conduct the study at Family Planning Victoria, and who did not receive compensation for this contribution; Vicky Reddick, Family Planning Victoria, and Erica Brown, Genetic Health Services Victoria, for their assistance in recruiting participants in Phase II, and who were employed for this task; Anna Flouris, Murdoch Childrens Research Institute, for assistance in the development of the brochure and questionnaires and who was employed for this task.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Metcalfe, S., Jacques, A., Archibald, A. et al. A model for offering carrier screening for fragile X syndrome to nonpregnant women: results from a pilot study. Genet Med 10, 525–535 (2008). https://doi.org/10.1097/GIM.0b013e31817c036e
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1097/GIM.0b013e31817c036e