Abstract
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in the developed world. Monthly or as-needed (PRN) dosing strategies of intravitreal ranibizumab have been established as efficacious treatment options for neovascular AMD. More recently, the ‘treat-and-extend’ dosing regimen (TREX) is being adopted in clinical practice as it represents a patient-centric and economical option, reducing treatment burden by extending injection intervals when possible. However, the efficacy of TREX using ranibizumab monotherapy remains to be defined. Therefore, we performed a systematic review to assess the current evidence for TREX using ranibizumab by searching MEDLINE, Embase and PubMed. Of the 1733 articles identified, nine TREX studies were included in our analysis (n=748 eyes). Average patient age was 79.25 (range: 77.34–82.00; SD: 7.27). Baseline BCVA ranged from 48.5–68.9 ETDRS letters. BCVA improvement was 8.92 letters at 1 year (range: 6.5–11.5; SD: 7.54), as a weighted mean accounting for numbers of study eyes. The weighted mean number of injections at one year was 8.60 (range: 7.3–12.0; SD: 1.73). Previously, the landmark ANCHOR and MARINA trials reported gains of 11.3 and 7.2 letters, respectively, using monthly ranibizumab. Chin-Yee et al reported a gain of 3.5 ETDRS letters with 5.3 (S.D. 0.66) PRN ranibizumab injections as weighted means at 1 year in their recent systematic review. Our analysis suggests that TREX delivers visual outcomes superior to PRN and approaches similar efficacy to monthly injections. Further RCTs are needed to fully evaluate the efficacy and economy of TREX in the long-term.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is a progressive, degenerative disease of the retina that causes central vision loss.1 It is the leading cause of blindness in the elderly in developed nations.2 AMD is classified as dry or neovascular (wet) based on the absence or presence of new blood vessels that have invaded the retina, respectively.3 Neovascular AMD affects 10–15% of AMD patients.4 In the United Kingdom (UK), over 338000 individuals in 2013 were affected by neovascular AMD, with 50000 cases resulting in blindness.5
Neovascular AMD is characterised by choroidal neovascularisation driven by vascular endothelial growth factor-A (VEGF-A), a signal protein that drives growth of morphologically fragile new vessels that tend to leak and haemorrhage, resulting in photoreceptor damage and vision impairment.6 Agents that antagonise VEGF-A decrease the accumulated fluid at the back of the eye and cause regression of the new fragile vessels. There are two Anti-VEGF agents currently licensed in Europe and approved by the National Institute for Health and Care Excellence (NICE) for the treatment of wet AMD in the UK: ranibizumab (Lucentis, Genentech (San Francisco, CA, USA)/Novartis, Basel, Switzerland) and aflibercept (Eylea, Bayer, Leverkusen, Germany).7 Ranibizumab is a recombinant, humanised, monoclonal, VEGF-specific antibody fragment. Regular monthly injections of ranibizumab are established as the gold standard treatment for neovascular AMD.8, 9, 10 A recent UK study estimated that the National Health Service (NHS) spent over £327 million on intravitreal use of ranibizumab in the year 2015, £212 million on aflibercept and £246000 on bevacizumab (Avastin, Roche, Basel, Switzerland), an unlicensed anti-VEGF agent also used for the treatment of neovascular AMD, totalling over £539 million.7, 11
Until recently, the licensed dosing regimen for the treatment of neovascular AMD using ranibizumab in Europe involved fixed monthly dosing until maximum VA is achieved, followed by monitoring and treatment intervals determined by the ophthalmologist pro-re-nata (PRN) based on disease activity.12 The clinical workload associated with the multiple follow-ups required with this treatment strategy is significant. Tufail et al demonstrated that ongoing capacity issues at AMD Clinics in the UK have prevented those departments from maintaining the regular monitoring visits, leading to delays in patients’ follow-up appointments and treatments, with consequential loss of vision.13 In addition, the VISION 2020 UK Macular Interest Group Survey revealed that necessary staffing to deliver neovascular AMD treatment is significantly below expected levels and demand far exceeds capacity.14 Moreover, key trials for PRN ranibizumab dosing have demonstrated a wide variability in the number of injections required by patients over time, suggesting heterogeneity in disease reactivation intervals between patients and supporting the need for alternative, individualised treatment regimens.15, 16, 17
The ‘treat-and-extend’ (TREX) dosing regimen is a strategy that aims to resolve macular exudation and maintain the macula in a ‘dry state’ with, where possible, fewer patient visits for investigation and treatment as compared to monthly dosing.18 The regimen involves an initial loading sequence of at least three monthly injections.19 As long as visual acuity is stable, treatment intervals are gradually increased. The maximal safe interval is not known. Some authors recommend a maximum of 10 weeks19 while others recommend 12 weeks.20 If there are any changes, treatment intervals are shortened by 2 weeks. TREX dosing therefore offers a practical solution to reduce treatment burden associated with multiple follow-up appointments. Furthermore, TREX may represent a more cost effective therapeutic option as compared to regular dosing. ‘As-needed’ or pro-re-nata (PRN) dosing involves initially giving regular injections, typically for a minimum of three months, followed by routine follow-up and only further injections given reactively when there is evidence of changes.21 The TREX regimen offers a proactive, structured treatment protocol, whereas a PRN regimen does not represent a structured option; patients are treated reactively waiting for symptoms and signs of activity to appear.
The TREX dosing regimen using ranibizumab monotherapy has not yet been assessed in a systematic review. The aim of this systematic review is to assess and compare the effectiveness of the TREX-dosing regimen for intravitreal ranibizumab, with PRN and regular dosing strategies for the treatment of neovascular AMD.
Materials and methods
Search strategy
We entered the medical subject headings (MeSH) terms ‘macular degeneration’, ‘AMD’, ‘ranibizumab’ and ‘Lucentis’ into the following search platforms: the Cochrane Central Register of Controlled Trials (CENTRAL) (including the Cochrane Eyes and Vision Group (CEVG) Trials Register), Ovid MEDLINE(R) (1946 to present), Ovid MEDLINE In-Process and Other Non-Indexed Citations, EMBASE Classic+Embase (1947 to present) and PubMed (1948 to present). We uploaded our search results onto EndNote X7 (Thomson Reuters, New York, NY) reference management software. No date or language restrictions were used in the electronic search for papers. Appendix 1 includes full details of keywords and MeSH terms used. We included Level IV evidence and above, i.e. case series, cohort studies, case-control studies, randomised controlled trials (RCTs) and systematic reviews, as defined by the Oxford Centre for Evidence-based Medicine.22
We employed a three-stage screening process first assessing titles, followed by abstracts, then full papers. Our screening questions are included in Appendix 2. Two investigators independently screened the studies delivered by the above search strategy using the screening questions, classifying the studies as ‘include’, ‘exclude’ or ‘unclear’. In the event of studies assessed as ‘unclear’ after full text screening due to ambiguous or missing information, the study authors would be contacted for clarification. For any discrepancies arising between the two investigators, a senior author volunteered to act as the third arbitrator to make the final judgement.
Outcome measures
The primary outcome measure was the mean change in best-corrected visual acuity (BCVA) at 1 year, using Early Treatment Diabetic Retinopathy Score (ETDRS) letters. A single-arm meta-analysis was performed by pooling the data and computing a weighted mean to account for the number of eyes per study. Secondary outcome measures included the weighted mean number of injections required and cost analyses of treatment regimes. The unit of analysis was the enrolled study eye of the participant.
Data extraction and synthesis
The following data were extracted from each study:
-
1
Study design
-
2
Study location
-
3
Number of eyes enrolled
-
4
Follow-up in months
-
5
Mean age in years
-
6
Number of injections
-
7
Baseline BCVA in ETDRS letters
-
8
BCVA at 12 months in ETDRS letters
We calculated the total number of eyes enrolled across all studies that received ranibizumab monotherapy. Where BCVA was recorded in Snellen or LogMAR, we employed the Gregori et al method to convert to ETDRS letters to facilitate comparison and to the data synthesis.23
Results
Search results
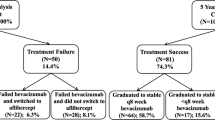
Figure 1 is a PRISMA flowchart summarising our screening process. Of 1733 studies identified, full data for mean BCVA improvement and mean number of injections at 1 year were obtained for nine studies comprising a total of 748 eyes. Two papers did not assess the treat-and-extend dosing protocol for neovascular AMD using ranibizumab monotherapy and were therefore excluded. Out of the 10 studies qualifying for our review, full data for number of injections and BCVA with ranibizumab monotherapy were not obtained from the study authors for one study and therefore could not be included.
Out of the nine included studies, one was a RCT while the remaining eight were observational in nature, thus providing only low-to-moderate quality of evidence based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) classification.24 Thus, the data extraction and risk of bias assessment was conducted solely for the RCT in the first instance.
Primary and secondary outcomes of RCT
This was a phase IIIb, multicenter, randomised, controlled clinical trial conducted in the United States of America. Average patient age was 77 years (range: 59–96). Mean baseline BCVA was 60 ETDRS letters. Fifty-seven eyes (95%) completed the full 12-month follow-up. Mean BCVA improved by 9.2 and 10.5 letters in the monthly and TREX cohorts, respectively (P=0.60). The mean number of injections given through month 12 was 13.0 and 10.1 (range: 7.0–13.0) in the monthly and TREX cohorts respectively (P<0.0001).
Risk of bias assessment for RCT
The Cochrane Collaboration’s tool for assessing risk of bias was used.25 Overall, this RCT was judged as low risk of bias.
The allocation sequence generation method was described in sufficient detail to demonstrate it should produce comparable groups. The method to conceal the allocation sequence was described in sufficient detail to demonstrate that intervention allocations could not have been foreseen in advance of, or during, enrolment. Thus, the risk of selection bias was low.
The nature of this RCT rendered it difficult to blind study participants and staff from knowledge of which intervention a participant received, as the intervention protocols for the monthly and TREX cohorts were different. For similar reasons, it would have been challenging to blind outcome assessors from knowledge of the allocated interventions. However, neither were stated explicitly. Therefore, the risks of performance and detection biases are unclear.
A 95% follow-up was achieved at 1 year with reasons for withdrawals stated. Intention-to-treat analysis was used. Numbers were reported per intervention arm. Therefore, the risk of attrition bias was low.
All relevant clinical outcomes were fully reported. There was no evidence of the possibility of selective outcome reporting. Therefore, the risk of reporting bias was low.
Primary and secondary outcomes including observational studies
The data from all nine studies were pooled to compute average means across the studies. Average patient age was 79.25 (range: 77.34–82.00; SD: 7.27). Baseline BCVA ranged from 48.5–68.9 ETDRS letters. BCVA improvement was 8.92 letters at 1 year (range: 6.5–11.5; SD: 7.54), as a weighted mean accounting for numbers of study eyes. The weighted mean number of injections at 1 year was 8.60 (range: 7.3–12.0; SD 1.73). This represents a 26.7% reduction in frequency of injections as compared to monthly injections. No study performed a cost analysis of the treat-and-extend protocol for their patient cohort.
Table 1 is a summary of study characteristics for all nine studies, and Table 2 is a summary of the pooled data. Although the full data for BCVA improvement and mean number of injections were obtained for all nine studies, BCVA SD were not obtained for studies 5–8, nor the patient age data for study 7. Therefore, SDs for BCVA improvement were imputed by averaging the available SDs per metric, as per the Cochrane Handbook.25
Discussion
To our knowledge, this study represents the first systematic review of the TREX-dosing regimen using ranibizumab monotherapy in the treatment of neovascular AMD. Conventional meta-analysis was not possible due to only one TREX RCT having been identified. However, both the RCT and the pooled data demonstrate that TREX can reduce the frequency of injections while improving vision. Therefore, TREX represents a viable solution in tackling the tremendous treatment burden associated with monthly ranibizumab injections.
Our pooled results demonstrate a gain of 8.92 (SD: 7.54) ETDRS letters as a weighted mean at 1 year, with 8.60 (SD: 1.73) injections as a weighted mean at 1 year, across nine TREX studies (n=748 eyes). Out of the nine studies, only one was an RCT comparing TREX to monthly dosing, performed by Wycoff et al.26 This was a phase IIIb, multicentre RCT involving a relatively small cohort of 60 patients randomised 1:2 to monthly and TREX management, respectively. Fifty-seven eyes (95%) completed month 12, at which point mean BCVA improved by 9.2 and 10.5 letters in the monthly and TREX cohorts, respectively (P=0.60), demonstrating non-inferiority in visual outcomes. The mean number of injections administered through month 12 was 13.0 and 10.1 in the monthly and TREX cohorts, respectively. Due to the lack of RCTs, conventional meta-analysis could not be performed to ascertain whether TREX delivers comparable visual outcomes compared to monthly and PRN dosing.
Chin-Yee et al performed a systematic review of the TREX regimen vs PRN dosing for neovascular AMD, having computed their search in 2013.27 Their systematic review demonstrated a gain of 9.17 (SD: 3.8) ETDRS letters as a weighted mean at 1 year, with 8.34 (SD: 0.66) injections as a weighted mean at one year, across eight TREX studies (n=1073 eyes). However, the key difference in their study method was the inclusion of patients receiving bevacizumab, presuming its efficacy to be equivalent to ranibizumab. Only four of the studies published at the time of their systematic review included ranibizumab monotherapy. Our study benefitted from a larger number of published TREX studies available. Additionally, we endeavoured to obtain results pertaining to patients receiving ranibizumab monotherapy by corresponding with study authors. Despite these differences in study methods, our study findings for TREX using ranibizumab monotherapy substantiate those of Chin-Yee et al. Furthermore, Chin-Yee et al additionally systematically reviewed studies of patients with neovascular AMD receiving ranibizumab or bevacizumab PRN, demonstrating a gain of 3.5 (SD: 4.5) ETDRS letters as a weighted mean at 1 year, with 5.3 (S.D. 0.66) injections as a weighted mean at 1 year, across 62 PRN studies (10716 eyes). They demonstrated this change in BCVA was significantly lower than that in TREX (Mann–Whitney’s test; P=0.0006).
The landmark ANCHOR and MARINA trials assessing monthly intravitreal ranibizumab for neovascular AMD demonstrated mean gain in BCVA of 11.3 and 7.2 at 1 year, respectively.28, 29 Our systematic review demonstrates that a gain of 8.92 letters at 1 year can be achieved with TREX, but further RCTs for TREX are required for future meta-analysis to conclude that it delivers comparable outcomes to monthly dosing. Nevertheless, it is encouraging to find that our systematic review of TREX has demonstrated approximately the average of the ANCHOR and MARINA visual outcomes in real world settings.
Assuming further RCTs support the hypothesis that TREX delivers non-inferior clinical outcomes to monthly dosing, a cost comparison between the two strategies strengthens the case for wider adoption of TREX in the UK. Using the data from the UK National Health Service (NHS) National Tariff Payment System 2016/1730 and the British National Formulary (BNF),31 we have estimated that the mean annual cost of treating neovascular AMD with ranibizumab using the TREX regimen is £8287.80 per patient compared to £11545.00 annually for a patient receiving monthly intravitreal ranibizumab and monthly follow-up with OCT as per the ANCHOR and MARINA trial protocols. This equals annual saving of £3257.20, representing a cost reduction of 28.21%. These calculations assume that each TREX patient has an initial visit (£156) plus an average of 7.6 follow-up visits (£107), all including the cost of an OCT scan (£43), while patients receiving monthly doses also have monthly follow-up with OCT scans which would be highly challenging, given the tremendous associated treatment burden. Moreover, the calculation uses the BNF price of £742.00 per vial of ranibizumab, which may be confidentially negotiated to a lower price by the NHS. Nonetheless, this cost comparison demonstrates a substantial saving can be achieved by adopting the TREX regimen.
This study has a number of limitations. Crucially, we were unable to perform conventional meta-analysis with a consistent comparator across the studies. In addition to the majority of studies being rated as low-to-moderate quality of evidence based on the GRADE classification, the pooled data demonstrating a weighted mean gain of 8.92 letters using the TREX regimen cannot be interpreted as causation. The pooled data are limited by the absence of overall randomisation leading to high risk of bias. This highlights a need for future RCTs to evaluate the efficacy of the TREX-dosing regimen vs PRN and monthly dosing, in order to statistically assess whether TREX delivers comparable clinical outcomes in the long term. Furthermore, this systematic review revealed the missing data relating to numbers of injections and visual outcomes in patients receiving ranibizumab monotherapy in several studies. This was because several studies only reported results for patients receiving both bevacizumab and ranibizumab. However, after contacting study authors, we obtained full data for numbers of injections and visual outcomes for patients receiving ranibizumab monotherapy and imputed the SD for BCVA improvement as this was missing for three studies. Finally, despite being unable to perform conventional meta-analysis, a strength of this systematic review was the inclusion of numerous real world outcome studies, demonstrating successful visual outcomes for TREX in real world settings.
References
Bowling B . Kanski’s Clinical Ophthalmology: A systematic approach. 8th edn. Saunders ltd: Philadelphia, PA, USA 2015.
Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PT et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004; 122 (4): 564–572.
Ambati J, Fowler BJ . Mechanisms of age-related macular degeneration. Neuron 2012; 75 (1): 26–39.
Wong TY, Chakravarthy U, Klein R, Mitchell P, Zlateva G, Buggage R et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology 2008; 115: 116–126.
Owen CG, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR . The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol. 2012; 96 (5): 752–756.
Ferrara N . VEGF-A: a critical regulator of blood vessel growth. Eur Cytokine Netw 2009; 20 (4): 158–163.
NICE. Guideline scope: Age-related macular degeneration: diagnosis and management. Available at: https://www.nice.org.uk/guidance/GID-CGWAVE0658/documents/final-scope (accessed 08 December 2016).
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355 (14): 1432–1444.
Brown DM, Heier JS, Ciulla T, Benz M, Abraham P, Yancopoulos G et al. Primary endpoint results of a phase II study of vascular endothelial growth factor trap-eye in wet age-related macular degeneration. Ophthalmology 2011; 118 (6): 1089–1097.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355 (14): 1419–1431.
Shalaby AK, Lewis K, Bush K, Meredith PR, Di Simplicio S, Lockwood AJ . Licence to save: a UK survey of anti-VEGF use for the eye in 2015. Eye 2016; 30: 1404–1406 [Online].
European Medicines Agency (EMA). Lucentis 10 mg/ml solution for injection. Summary of Product Characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000715/WC500043546.pdf (accessed 18 September 2016).
Tufail AXW, Johnston R, Akerele T, McKibbin M, Downey L, Natha S et al. The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology 2014; 121 (5): 1092–1101.
VISION 2020. VISION 2020. AMD Services Survey. 2012 Available at: http://www.vision2020uk.org.uk/amd-services-survey/ (accessed 08 December 2016.
Abraham P, Yue H, Wilson L . Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 2. Am J Ophthalmol 2010; 150 (3): 315–24.e1.
Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol 2008; 145 (2): 239–248.
Schmidt-Erfurth U, Eldem B, Guymer R, Korobelnik JF, Schlingemann RO, Axer-Siegel R et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology 2011; 118 (5): 831–839.
Spaide R . Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol 2007; 143: 679–680.
Engelbert M, Zweifel SA, Freund KB . ‘Treat and extend" dosing of intravitreal antivascular endothelial growth factor therapy for type 3 neovascularization/retinal angiomatous proliferation. Retina 2009; 29 (10): 1424–1431.
Berg K, Pedersen TR, Sandvik L, Bragadottir R . Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology 2015; 122 (1): 146–152.
Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Davis JL et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol 2009; 148: 43–58.
Oxford Centre for Evidence-based Medicine. Levels of Evidence. Available at: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ (accessed 09 October 2016).
Gregori NZ, Feuer W, Rosenfeld PJ . Novel method for analyzing snellen visual acuity measurements. Retina 2010; 30: 1046–1050.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926.
The Cochrane Collaboration. The Cochrane Handbook for Systematic Reviews of Interventions. 2011. Available at: http://handbook.cochrane.org/chapter_16/16_1_3_1imputing_standard_deviations.htm (accessed 12 December 2016).
Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L et al. Prospective Trial of Treat-and-Extend versus Monthly Dosing for Neovascular Age-Related Macular Degeneration: TREX-AMD 1-Year Results. Ophthalmology 2015; 122 (12): 2514–2522.
Chin-Yee D, Eck T, Fowler S, Hardi A, Apte RS . A systematic review of as needed versus treat and extend ranibizumab or bevacizumab treatment regimens for neovascular age-related macular degeneration. Br J Ophthalmol 2016; 100 (7): 914–917.
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T . ANCHOR Study Group. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology 2009; 116 (1): 57–65.e5.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et alMARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355 (14): 1419–1431.
NHS England. NHS National Tariff Payment System 2016/17. Available at: https://www.gov.uk/government/publications/nhs-national-tariff-payment-system-201617 (accessed 12 December 2016).
British National Formulary. Lucentis. Available at: https://www.evidence.nhs.uk/formulary/bnf/current/11-eye/118-miscellaneous-ophthalmic-preparations/1182-ocular-diagnostic-and-peri-operative-preparations-and-photodynamic-treatment/subfoveal-choroidal-neovascularisation/ranibizumab/lucentis (accessed 12 December 2016).
Abedi F, Wickremasinghe S, Islam AF, Inglis KM, Guymer RH . Anti-VEGF treatment in neovascular age-related macular degeneration: a treat-and-extend protocol over 2 years. Retina 2014; 34 (8): 1531–1538.
Calvo P, Abadia B, Ferreras A, Ruiz-Moreno O, Leciñena J, Torrón C . Long-term visual outcome in wet age-related macular degeneration patients depending on the number of ranibizumab injections. J Ophthalmol 2015; 2015: 820605.
Chen G, Li W, Tzekov R, Jiang F, Mao S, Tong Y . Bevacizumab versus ranibizumab for neovascular age-related macular degeneration: a meta-analysis of randomized controlled trials. Retina 2015; 35 (2): 187–193.
Mrejen S, Jung JJ, Chen C, Patel SN, Gallego-Pinazo R, Yannuzzi N et al. Long-term visual outcomes for a treat and extend anti-vascular endothelial growth factor regimen in eyes with neovascular age-related macular degeneration. J Clin Med 2015; 4 (7): 1380–1402.
Oubraham H, Cohen SY, Samimi S, Marotte D, Bouzaher I, Bonicel P et al. Inject and extend dosing versus dosing as needed: a comparative retrospective study of ranibizumab in exudative age-related macular degeneration. Retina 2011; 31 (1): 26–30.
Rayess N, Houston SK 3rd, Gupta OP, Ho AC, Regillo CD . Treatment outcomes after 3 years in neovascular age-related macular degeneration using a treat-and-extend regimen. Am J Ophthalmol 2015; 159 (1): 3–8.e1.
Toalster N, Russell M, Ng P . A 12-month prospective trial of inject and extend regimen for ranibizumab treatment of age-related macular degeneration. Retina 2013; 33 (7): 1351–1358.
Acknowledgements
We thank the authors of the included studies for providing further data where required for our study. SRR was supported by a UK National Institute for Health Research (NIHR) Academic Foundation post supervised by TK and BLS. HL was supported by a UK NIHR Academic Clinical Lectureship in ophthalmology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SRR, HA and AJL have been supported by travel grants from Bayer. The remaining authors declare no conflict of interests.
Additional information
Presentations: SRR, HA, HL, RP, BLS, TK, AJL. A systematic review to assess the ‘treat-and-extend’ dosing regimen compared to monthly and as-needed dosing for neovascular age-related macular degeneration using ranibizumab. Association for Research in Vision and Ophthalmology Annual Meeting. 2nd May 2016
Appendices
Appendix 1: Search terms
-
1
exp Macular degeneration/
-
2
AMD.tw.
-
3
(age-related adj3 maculopath*).tw.
-
4
(retina* adj3 degenerat*).tw.
-
5
(macula* or heredomacula*).tw.
-
6
(dystroph* adj3 (macula* or heredomacula*)).tw.
-
7
(age-related adj3 (macula* or heredomacula*)).tw.
-
8
(degenerat* adj3 (macula* or heredomacula*)).tw.
-
9
junius kuhnt.tw.
-
10
(atroph* adj3 (macula* or heredomacula*)).tw.
-
11
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
-
12
Ranibizumab.tw.
-
13
Lucentis.tw.
-
14
12 or 13
-
15
11 and 14
Search computed on 26 September 2015
Appendix 2: Screening questions
Stage 1: Title screening
Is the study Level IV evidence or above (including case series, cohort studies, case-control studies, randomised controlled trials and systematic reviews)?
Yes
No
Unclear
Is the study concerned with the use of ranibizumab in neovascular age-related macular degeneration?
Yes
No
Unclear
Stage 2: Abstract screening
Is the study concerned with variable dosing regimens for treatment of age-related macular degeneration?
Yes
No
Unclear
Stage 3: Full paper screening
Is the study concerned with variable dosing regimens featuring regular initial doses of ranibizumab followed by progressively longer treatment intervals, commonly referred to as ‘treat-and-extend’ dosing regimen and also known as ‘inject-and-extend’?
Yes
No
Unclear
Appendix 3
Rights and permissions
About this article
Cite this article
Rufai, S., Almuhtaseb, H., Paul, R. et al. A systematic review to assess the ‘treat-and-extend’ dosing regimen for neovascular age-related macular degeneration using ranibizumab. Eye 31, 1337–1344 (2017). https://doi.org/10.1038/eye.2017.67
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2017.67
This article is cited by
-
Efficacy, safety, and treatment burden of treat-and-extend versus alternative anti-VEGF regimens for nAMD: a systematic review and meta-analysis
Eye (2023)
-
A Delphi study on the clinical management of age-related macular degeneration
International Ophthalmology (2022)
-
Evaluation of efficacy and recurrence for anti-vascular endothelial growth factor therapy in idiopathic choroidal neovascularization
BMC Ophthalmology (2020)
-
Ranibizumab port delivery system (RPDS): realising long awaited dream of prolonged VEGF suppression
Eye (2020)
-
Action on neovascular age-related macular degeneration (nAMD): recommendations for management and service provision in the UK hospital eye service
Eye (2019)