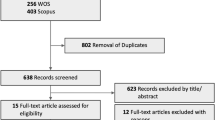
Abstract
Intravitreal injections of antiangiogenic agents are pivotal in treating neovascular age-related macular degeneration (nAMD). The comparative efficacy and safety of treat-and-extend (T&E) versus bimonthly, monthly, and pro re nata (PRN) dosing remains unclear. A systematic review and meta-analysis of English-language RCTs reporting on efficacy and/or safety outcomes of dosing regimens of anti-VEGF agents in nAMD was performed. Best-corrected visual acuity (BCVA, ETDRS letters) at last follow-up represented the primary endpoint, while central subfield thickness (CSFT, μm), injection burden, and ocular adverse events were secondary endpoints. A random effects meta-analysis was performed, and 95% confidence intervals were calculated. Across six RCTs, 781 T&E-, 663 monthly-, 130 PRN-, and 123 bimonthly treated eyes were included. Mean changes in BCVA and CSFT at last follow-up were similar between T&E versus monthly (WMD, –0.62 letters; 95% CI, –2.12 to 0.87; P = 0.41; WMD, 5.30 microns; 95% CI, –10.67 to 21.26; P = 0.52, respectively), bimonthly (WMD, 1.68 letters; 95% CI, –3.55 to 6.91; P = 0.53; WMD, –18.91 microns; 95% CI, –46.41 to 8.60; P = 0.18, respectively), and PRN (BCVA WMD, 1.08 letters; 95% CI, –2.95 to 5.11; P = 0.60) regimens. T&E was associated with a reduced injection burden versus monthly (WMD, –4.52 injections; 95% CI, –6.66 to 2.39; P < 0.001) but higher injection burden versus PRN (WMD, 1.81 injections; 95% CI, 1.12 to 2.51; P < 0.001) dosing. There was no significant difference in safety outcomes amongst comparators. There was no significant difference in efficacy and safety between T&E, bimonthly, monthly, and PRN dosing. T&E resulted in fewer injections versus monthly and fewer clinic visits versus PRN.
摘要
玻璃体腔内注射抗血管生成药物是新生血管性老年性黄斑变性(nAMD)非常重要的治疗方法。T&E方案与半个月给药、每月给药方案以及按需剂量 (PRN) 方案相比, 在疗效和安全性方面尚不清楚。我们对报道抗VEGF药物的治疗方案在nAMD治疗中的疗效和/或安全性评估的英文随机对照试验 (RCT) 进行了系统回顾和荟萃分析。最后一次随访的最佳矫正视力(BCVA, ETDRS字母)是主要的临床结局终点, 而视网膜中央子区厚度(CSFT, μm)、注射剂量负担和眼部不良事件是次要的结局终点。我们进行了随机效应荟萃分析, 并计算95%置信区间。在6个RCTs中, 781只眼为T&E治疗, 663只眼为每月治疗, 130只眼为PRN治疗, 123只眼为双月治疗。末次随访时, T&E方案分别与每月方案 (WMD, -0.62个字母; 95%CI, -2.12-0.87; P = 0.41; WMD, 5.30微米; 95%CI, -10.67-21.26; P = 0.52), 双月方案 (WMD, 1.68个字母; 95%CI, - 3.55 - 6.91; P = 0.53; WMD, -18.91微米; 95%CI, -46.41- 8.60; P = 0.18)和PRN方案 (BCVA WMD, 1.08个字母; 95%CI, -2.95-5.11; P = 0.60) 的BCVA和CSFT平均变化相比较均相似。 T&E方案的注射剂量负担低于每月方案(WMD, - 4.52次注射; 95%CI, -6.66-2.39; P < 0.001), 但高于PRN方案 (WMD, 1.81次; 95%CI, 1.12-2.51; P < 0.001)。比较组间安全性结果无显著差异。T&E、双月、每月和PRN给药方案之间的疗效和安全性无显著差异。与每月方案相比, T&E方案注射剂量更少; 与PRN方案相比, T&E方案的就诊次数更少。
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
References
Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988;32:375–413.
Congdon N, O'Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–85.
Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51.
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116.
Morris B, Imrie F, Armbrecht AM, Dhillon B. Age-related macular degeneration and recent developments: New hope for old eyes? Postgrad Med J. 2007;83:301–7.
Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72.
Klein R, Klein BEK, Linton KLP. Prevalence of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–43.
Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia: The Blue Mountains Eye Study. Ophthalmology. 1995;102:1450–60.
Weih LAM, VanNewkirk MR, McCarty CA, Taylor HR. Age-specific causes of bilateral visual impairment. Arch Ophthalmol. 2000;118:264–9.
Muether PS, Hermann MM, Koch K, Fauser S. Delay between medical indication to anti-VEGF treatment in age-related macular degeneration can result in a loss of visual acuity. Graefe’s Arch Clin Exp Ophthalmol. 2011;249:633–7.
Bourne RRA, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, et al. Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob Health. 2013;1:339–49.
Gale RP, Mahmood S, Devonport H, Patel PJ, Ross AH, Walters G, et al. Action on neovascular age-related macular degeneration (nAMD): recommendations for management and service provision in the UK hospital eye service. Eye. 2019;33:1–21.
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2006;116:57–65.e5.
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201.
Ng EWM, Adamis AP. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can J Ophthalmol. 2005;40:352–68.
Kim LA, D’Amore PA. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am J Pathol. 2012;181:376–9.
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7.
Fogli S, Del Re M, Rofi E, Posarelli C, Figus M, Danesi R. Clinical pharmacology of intravitreal anti-VEGF drugs. Eye. 2018;32:1010–20.
Moutray T, Chakravarthy U. Age-related macular degeneration: current treatment and future options. Ther Adv Chronic Dis. 2011;2:325–31.
Rosenfeld PJ. Bevacizumab versus ranibizumab for AMD. N Engl J Med. 2011;364:1966–7.
Emerson MV, Lauer AK. Emerging therapies for the treatment of neovascular age-related macular degeneration and diabetic macular edema. BioDrugs. 2007;21:245–57.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Payne JF, Wykoff CC, Clark WL, Bruce BB, Boyer DS, Brown DM, et al. Randomized trial of treat and extend ranibizumab with and without navigated laser versus monthly dosing for diabetic macular edema: TREX-DME 2-year outcomes. Am J Ophthalmol. 2019;202:91–99.
Silva R, Berta A, Larsen M, Macfadden W, Feller C, Monés J, et al. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology. 2018;125:57–65.
Pilli S, Kotsolis A, Spaide RF, Slakter J, Freund KB, Sorenson J, et al. Endophthalmitis associated with intravitreal anti-vascular endothelial growth factor therapy injections in an office setting. Am J Ophthalmol. 2008;145:879–82.
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–83.
Holz FG, Amoaku W, Donate J, Guymer RH, Kellner U, Schlingemann RO, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: The SUSTAIN study. Ophthalmology. 2011;118:663–71.
Busbee BG, Ho AC, Brown DM, Heier JS, Suñer IJ, Li Z, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120:1046–56.
Singer MA, Awh CC, Sadda S, Freeman WR, Antoszyk AN, Wong P, et al. HORIZON: An open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119:1175–83.
Amoaku W, Balaskas K, Cudrnak T, Downey L, Groppe M, Mahmood S, et al. Initiation and maintenance of a treat-and-extend regimen for ranibizumab therapy in wet age-related macular degeneration: Recommendations from the UK Retinal Outcomes Group. Clin Ophthalmol. 2018;12:1731–40.
Ross AH, Downey L, Devonport H, Gale RP, Kotagiri A, Mahmood S, et al. Recommendations by a UK expert panel on an aflibercept treat-and-extend pathway for the treatment of neovascular age-related macular degeneration. Eye. 2020;34:1825–34.
Spaide R. Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol. 2007;143:679–80.
U.S. National Library of Medicine. Prospective OCT study with Lucentis for neovascular AMD (PrONTO study) (Clinicaltrials.gov Identifier NCT00344227). 2006. https://clinicaltrials.gov/ct2/show/NCT00344227.
Kertes PJ, Galic IJ, Greve M, Williams RG, Rampakakis E, Scarino A, et al. Canadian Treat-and-Extend Analysis Trial with Ranibizumab in patients with neovascular age-related macular disease: one-year results of the randomized Canadian Treat-and-Extend Analysis Trial with Ranibizumab study. Ophthalmology. 2019;126:841–8.
Engelbert M, Zweifel SA, Freund KB. “Treat and extend” dosing of intravitreal antivascular endothelial growth factor therapy for type 3 neovascularization/retinal angiomatous proliferation. Retina. 2009;29:1424–31.
Augsburger M, Sarra GM, Imesch P. Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: a comparative study. Graefe’s Arch Clin Exp Ophthalmol. 2019;257:1889–95.
Freund KB, Korobelnik JF, Devenyi R, Framme C, Galic J, Herbert E, et al. Treat-and-extend regimen with anti-VEGF agents in retinal diseases: a literature review and consensus recommendations. Retina. 2015;35:1489–506.
Lanzetta P, Loewenstein A, The Vision Academy Steering Committee. Fundamental principles of an anti-VEGF treatment regimen: Optimal application of intravitreal anti–vascular endothelial growth factor therapy of macular diseases. Graefe’s Arch Clin Exp Ophthalmol. 2017;255:1259–73.
Gupta OP, Shienbaum G, Patel AH, Fecarotta C, Kaiser RS, Regillo CD. A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration: clinical and economic impact. Ophthalmology. 2010;117:2134–40.
Okada M, Kandasamy R, Chong EW, McGuiness M, Guymer RH. The treat-and-extend injection regimen versus alternate dosing strategies in age-related macular degeneration: a systematic review and meta-analysis. Am J Ophthalmol. 2018;192:184–97.
Hatz K, Prünte C. Treat and extend versus pro re nata regimens of ranibizumab in neovascular age-related macular degeneration: A comparative 12 Month study. Acta Ophthalmol. 2017;95:e67–e72.
Oubraham H, Cohen SY, Samimi S, Marotte D, Bouzaher I, Bonicel P, et al. Inject and extend dosing versus dosing as needed: A comparative retrospective study of ranibizumab in exudative age-related macular degeneration. Retina. 2011;31:26–30.
Eichenbaum DA, Duerr E, Patel HR, Pollack SM. Monthly versus treat-and-extend ranibizumab for diabetic macular edema: a prospective, randomized trial. Ophthalmic Surg Lasers Imaging Retina. 2018;49:e191–e197.
Haga A, Kawaji T, Ideta R, Inomata Y, Tanihara H. Treat-and-extend versus every-other-month regimens with aflibercept in age-related macular degeneration. Acta Ophthalmol. 2018;96:e393–e398.
Kertes PJ, Galic IJ, Greve M, Williams G, Baker J, Lahaie M, et al. Efficacy of a treat-and-extend regimen with ranibizumab in patients with neovascular age-related macular disease: a randomized clinical trial. JAMA. Ophthalmol. 2020;138:244–50.
Scott IU, VanVeldhuisen PC, Ip MS, Blodi BA, Oden NL, Altaweel M, et al. Comparison of monthly vs treat-and-extend regimens for individuals with macular edema who respond well to anti-vascular endothelial growth factor medications: Secondary outcomes from the SCORE2 randomized clinical trial. JAMA Ophthalmol. 2018;136:337–45.
Ehlers JP, Wang K, Singh RP, Babiuch AS, Schachat AP, Yuan A, et al. A prospective randomized comparative dosing trial of ranibizumab in bevacizumab-resistant diabetic macular edema: the REACT Study. Ophthalmol Retin. 2018;2:217–24.
Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583.
Cochrane handbook for systematic reviews of interventions. Version 6.3, 2022. The Cochrane Collaboration; 2011.
Sterne J, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:1–8.
Babic A, Vuka I, Saric F, Proloscic I, Slapnicar E, Cavar J, et al. Overall bias methods and their use in sensitivity analysis of Cochrane reviews were not consistent. J Clin Epidemiol. 2020;119:57–64.
Babic A, Tokalic R, Amílcar Silva Cunha J, Novak I, Suto J, Vidak M, et al. Assessments of attrition bias in Cochrane systematic reviews are highly inconsistent and thus hindering trial comparability. BMC Med Res Methodol. 2019;19:1–10.
Wong H, Ouyang Y, Karim ME. The randomization-induced risk of a trial failing to attain its target power: assessment and mitigation. Trials. 2019;20:1–8.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Gil P, Gil J, Oliveira N, Laíns I, Camilo E, Fonseca C, et al. Influence of the vitreoretinal interface on the treatment with anti-VEGF for exudative age-related macular degeneration. Ophthalmologica. 2018;240:29–36.
López Gálvez MI, Arias Barquet L, S Figueroa M, García-Layana A, Ruiz Moreno JM, In-Eye Study G. Bimonthly, treat-and-extend and as-needed ranibizumab in naïve neovascular age-related macular degeneration patients: 12-month outcomes of a randomized study. Acta Ophthalmol. 2020;98:1–10. https://doi.org/10.1111/aos.14399.
Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L, et al. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology. 2015;122:2514–22.
Wykoff CC, Ou WC, Brown DM, Croft DE, Wang R, Payne JF, et al. Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: 2-year results of the TREX-AMD study. Ophthalmol Retin. 2017;1:314–21.
Wykoff CC, Ou WC, Croft DE, Payne JF, Brown DM, Clark WL, et al. Neovascular age-related macular degeneration management in the third year: Final results from the TREX-AMD randomised trial. Br J Ophthalmol. 2018;102:460–4.
Saenz-De-Viteri M, Recalde S, Fernandez-Robredo P, López Gálvez MI, Arias Barquet L, Figueroa MS, et al. Role of intraretinal and subretinal fluid on clinical and anatomical outcomes in patients with neovascular age‐related macular degeneration treated with bimonthly, treat‐and‐extend and as‐needed ranibizumab in the In‐Eye study. Acta Ophthalmol. 2021;99:1–10. https://doi.org/10.1111/aos.14786.
Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract. 1995;41:169–75.
McLenon J, Rogers MAM. The fear of needles: a systematic review and meta-analysis. J Adv Nurs. 2019;75:30–42.
Wright S, Yelland M, Heathcote K, Ng SK, Wright G. Fear of needles: Nature and prevalence in general practice. Aust Fam Physician. 2009;38:172–6.
Kim LN, Mehta H, Barthelmes D, Nguyen V, Gillies MC. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina. 2016;36:1418–31.
Chaudhary V, Gusenbauer K, Mak M, Barbosa J, Mohammad Mohaghegh P S, Popovic M. Waiting room educational media effect on preinjection anxiety for initial intravitreal injections. Can J Ophthalmol. 2016;51:71–75.
Rayat JS, Grewal PS, Whelan J, Tennant MTS, Choudhry N. Canadian preference and trends survey results for anti-VEGF treatment of macular edema. Can J Ophthalmol. 2016;51:233–7.
Patented Medicine Prices Review Board. The cost of drugs for age-related macular degeneration in Canada and internationally. 2018. https://www.canada.ca/content/dam/pmprb-cepmb/documents/reports-and-studies/annual-report/2018/PMPRB-annual-report-2018-en.pdf.
Ross EL, Hutton DW, Stein JD, Bressler NM, Jampol LM, Glassman AR, et al. Cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema: Analysis from the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial. JAMA Ophthalmol. 2016;134:888–96.
Radhakrishnan, K, Vincent A, Joseph RR, Moreno M, Dickescheid A, Agrawal R, et al. Hollow microcapsules as periocular drug depot for sustained release of anti-VEGF Protein. Pharmaceutics. 2019;11:330.
Robbie SJ, Lundh von Leithner P, Ju M, Lange CA, King AG, Adamson P, et al. Assessing a novel depot delivery strategy for noninvasive administration of VEGF/PDGF RTK inhibitors for ocular neovascular disease. Investig Ophthalmol Vis Sci. 2013;54:1490–1500.
Tsujinaka H, Fu J, Shen J, Yu Y, Hafiz Z, Kays J, et al. Sustained treatment of retinal vascular diseases with self-aggregating sunitinib microparticles. Nat Commun. 2020;11:330.
Borras L, Lenherr-Frey D, Grimshaw J, Rich-le P, Tietz J, Spohn G, et al. Brolucizumab, a unique single-chain antibody fragment inhibitor of vascular endothelial growth factor. Submitted for publication.
Holz FG, Dugel PU, Weissgerber G, Hamilton R, Silva R, Bandello F, et al. Single-chain antibody fragment VEGF inhibitor RTH258 for neovascular age-related macular degeneration. Ophthalmology. 2016;123:1080–9.
Tadayoni R, Sararols L, Weissgerber G, Verma R, Clemens A, Holz FG. Brolucizumab: a newly developed anti-VEGF molecule for the treatment of neovascular age-related macular degeneration. Ophthalmologica. 2021;244:93–101.
Novartis. Novartis reports one year results of Phase III MERLIN study evaluating Beovu® every four week dosing and provides update on Beovu clinical program. 2021. https://www.novartis.com/news/media-releases/novartis-reports-one-year-results-phase-iii-merlin-study-evaluating-beovu-every-four-week-dosing-and-provides-update-beovu-clinical-program.
Novartis. Data on file. MERLIN First interpretable results. 2021. https://www.novartis.com/news/media-releases/novartis-reports-one-year-results-phase-iii-merlin-study-evaluatingbeovu-every-four-week-dosing-and-provides-update-beovu-clinical-program.
Novartis. Novartis receives FDA approval for Beovu®, offering wet AMD patients vision gains and greater fluid reductions vs aflibercept. 2019. https://www.novartis.com/news/media-releases/novartis-receives-fda-approval-beovu-offering-wet-amd-patients-vision-gains-and-greater-fluid-reductions-vs-aflibercept.
U.S. National Library of Medicine. A study to evaluate the efficacy and safety of faricimab in participants with neovascular age-related macular degeneration (LUCERNE). 2019. https://clinicaltrials.gov/ct2/show/NCT03823300.
U.S. National Library of Medicine. A Study to Evaluate the Efficacy and Safety of Faricimab in Participants With Neovascular Age-Related Macular Degeneration (TENAYA) (Clinicaltrials.gov Identifier NCT03823287). 2019. https://clinicaltrials.gov/ct2/show/NCT03823287.
Genetech. FDA accepts application for Genentech’s Faricimab for the treatment of wet age-related macular degeneration (AMD) and diabetic macular edema (DME). 2021. https://www.gene.com/media/press-releases/14923/2021-07-28/fda-accepts-application-for-genentechs-f .
Campochiaro PA, Marcus DM, Awh CC, Regillo C, Adamis AP, Bantseev V, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2019;126:1141–54.
F. Hoffmann-La Roche Ltd. FDA accepts application for Roche’s Port Delivery System with ranibizumab (PDS) for treatment of neovascular or “wet” age-related macular degeneration (nAMD). 2021. https://www.roche.com/media/releases/med-cor-2021-06-24.htm.
Holekamp NM, Campochiaro PA, Chang MA, Miller D, Pieramici D, Adamis AP, et al. Archway randomized phase 3 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;129:295–307. https://doi.org/10.1016/j.ophtha.2021.09.016.
Gillies MC, Hunyor AP, Arnold JJ, Guymer RH, Wolf S, Pecheur FL, et al. Macular atrophy in neovascular age-related macular degeneration: A randomized clinical trial comparing ranibizumab and aflibercept (RIVAL Study). Ophthalmology. 2020;127:198–210.
Cho HJ, Yoo SG, Kim HS, Kim JH, Kim CG, Lee TG, et al. Risk factors for geographic atrophy after intravitreal ranibizumab injections for retinal angiomatous proliferation. Am J Ophthalmol. 2015;159:285–92.e1.
Grunwald JE, Pistilli M, Ying GS, Maguire MG, Daniel E, Martin DF, et al. Growth of geographic atrophy in the Comparison of Age-related macular degeneration Treatments Trials (CATT). Ophthalmology. 2015;122:809–16.
Kuroda Y, Yamashiro K, Ooto S, Tamura H, Oishi A, Nakanishi H, et al. Macular atrophy and macular morphology in aflibercept-treated neovascular age-related macular degeneration. Retina. 2018;38:1743–50.
Bailey C, Scott LJ, Rogers CA, Reeves BC, Hamill B, Peto T, et al. Intralesional macular atrophy in anti–vascular endothelial growth factor therapy for age-related macular degeneration in the IVAN trial. Ophthalmology. 2019;126:75–86.
Chakraborty D, Mondal S, Parachuri N, Kumar N, Sharma A. Brolucizumab—early experience with early extended interval regime in chronic centre involved diabetic macular oedema. Eye. 2021;36:358–60. https://doi.org/10.1038/s41433-021-01816-3.
Lam WC, Choudhry N, Wong D. Polypoidal choroidal vasculopathy in Canada. Can J Ophthalmol. 2020;55:199–211.
Fujiwara K, Yasuda M, Hata J, Oshima Y, Hashimoto S, Yoshitomi T, et al. Prevalence and risk factors for polypoidal choroidal vasculopathy in a general Japanese population: the Hisayama Study. Semin Ophthalmol. 2018;33:813–9.
Maberley DA, Hollands H, Chuo J, Tam G, Konkal J, Roesch M, et al. The prevalence of low vision and blindness in Canada. Eye. 2006;20:341–6.
Acknowledgements
We thank Dr. Rufino Silva on behalf of Novartis Pharma AG for providing supplementary unpublished data from the TREND 2018 trial, Novartis Pharmaceuticals Canada Inc. for providing supplementary unpublished data from the CANTREAT 2019 trial, and Dr. Charles C. Wykoff and Hannah Yu on behalf of Genentech, Inc. for providing supplementary unpublished data from the TREX-AMD 2015 trial.
Author information
Authors and Affiliations
Contributions
All authors have sufficiently fulfilled the four criteria of authorship as detailed by the 2019 International Committee of Medical Journal Editors (ICMJE) Recommendations.
Corresponding author
Ethics declarations
Competing interests
PN, ASD, AP: None. MMP: Physicians’ Services Incorporated (PSI) Foundation (RI). RHM: Allergan (C), Bayer (C, RI), Novartis (C, RI), Roche (C). PJK: Alcon (C), Allergan (RI), ArcticDx (E), Bayer (C, RI, RP), Novartis (C, RI, RP), Novelty Nobility (C), Roche (RI). Legend: C – consultant/consulting fees; E – equity owner; S – speaker honoraria; RI – research grant/financial support (to institution); RP – research grant/financial support (personal)
Ethics
The paper is exempt from ethical committee approval given that it is a meta-analysis of previously published data as opposed to a primary study using direct/individual patient data. This research in its entirety complies with the guidelines for human studies and should include evidence that the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nichani, P.A.H., Popovic, M.M., Dhoot, A.S. et al. Treat-and-extend dosing of intravitreal anti-VEGF agents in neovascular age-related macular degeneration: a meta-analysis. Eye 37, 2855–2863 (2023). https://doi.org/10.1038/s41433-023-02439-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02439-6