Abstract
Purpose
To determine whether the concentrations of vascular endothelial growth factor (VEGF) and monocyte chemoattractant protein (MCP)-1 in the vitreous changed after vitrectomy in patients with proliferative diabetic retinopathy (PDR).
Participants
Twenty-one eyes of 21 patients who needed a second surgery for PDR were included. The reasons for the second surgery were tractional retinal detachment (TRD), neovascular glaucoma, persistent vitreous hemorrhage, macular pucker, and secondary intraocular lens (IOL) implant.
Methods
We measured the VEGF and MCP-1 levels using sandwich enzyme-linked immunosorbent assays in vitreous samples collected from patients with PDR before pars plana vitrectomy (without IOL implantation), and from the same patients during the second surgery.
Results
There was not significant change in mean VEGF concentrations when comparing first (0.81±0.88 ng/ml) and second surgeries (1.09±1.51 ng/ml). The MCP-1 level was significantly elevated at the time of second surgery (2.20±2.21 ng/ml) compared with the first vitrectomy (0.72±0.57 ng/ml). The MCP-1 levels of the second surgery cases with TRD (3.18±2.27 ng/ml) increased significantly compared with those with other complications (1.72±2.10 ng/ml).
Conclusions
At the second vitrectomy, VEGF did not change significantly in the vitreous of the patients examined. The MCP-1 concentration was markedly elevated at the second vitrectomy, implying an association between the prolonged inflammation after vitrectomy and complications, especially TRD.
Similar content being viewed by others
Introduction
Proliferative diabetic retinopathy (PDR) is a leading cause of severe vision loss in people living in developed countries. Treatment can reduce the visual loss, but it is not always accessible or successful. Late complications of PDR are vitreous hemorrhage, neovascular glaucoma (NVG), tractional retinal detachment (TRD), combined tractional and rhegmatogenous retinal detachment, and severe fibrovascular proliferation. Pars plana vitrectomy (PPV) is the only treatment in these types of advanced PDR. The main objectives of the vitrectomy are to remove medium opacities, relieve all tractional adhesions, and manage recurrent complications from the previous vitrectomy. After the landmark study by Aiello et al1 on the association between retinal neovascularization and vascular endothelial growth factor (VEGF), the clearance of small-cell-signaling protein molecules was added to the objectives to reduce diabetic retinopathy after vitrectomy. Ongoing developments in vitreoretinal surgical instrumentation and techniques offer the potential advantages of reduced operating time and faster post-operative recovery. However, these systems still have major limitations when treating advanced PDR. According to recent reports, the incidence of reoperation after the first vitrectomy ranged from 7 to 22%.2, 3 The main causes of reoperations are recurrent vitreous cavity hemorrhage (early or delayed), TRD, and NVG.4, 5
Vitrectomy is an invasive procedure that leads to inflammation in the eyes. The inflammation after vitrectomy probably results from a complex interaction between several inflammatory factors. Anti-inflammatory eyedrops including corticosteroids are now used to manage inflammation after vitrectomy, although little is known about how long the inflammation continues after the surgery. Monocyte chemoattractant protein (MCP)-1 is a small cytokine belonging to the CC chemokine family that recruits monocytes, memory T cells, and dendritic cells to sites of tissue injury, infection, and inflammation.6, 7 It is also induced by hypoxia and bleeding.8, 9, 10, 11
Clinical studies revealed that MCP-1 increased in the vitreous of patients with PDR and it was suspected of contributing to the progression of diabetic retinopathy.9, 11, 12
To investigate how vitrectomy affects vitreous levels of VEGF and MCP-1, vitreous samples were obtained at the time of the first PPV and secondary intraocular lens (IOL) implantation or second vitrectomy in patients with PDR. The vitreous concentrations of VEGF/MCP-1 were compared between the first and second surgeries, and their relationship with complications developing after vitrectomy was also evaluated to clarify the mechanism of the prolonged activity of PDR after surgery.
Patients and methods
Patients who required vitreous surgery for PDR at the Fukuoka University Chikushi Hospital were fully informed of the procedures to be used and invited to participate in our study beginning in June 2007. All patients gave informed consent before inclusion in the study.
This prospective study recruited patients with type 2 diabetes mellitus with high-risk PDR requiring PPV, but with no history of triamcinolone or anti-VEGF antibody treatment during this study. PPV was indicated in high-risk PDR with persistent vitreous hemorrhage (PVH) or TRD. The status and changes in PDR were also determined using the modified Fukuda classification.13 From this classification, retinal hemorrhage, vitreous hemorrhage, fibrovascular membrane, maculopathy, TRD, and NVG were referenced, and vitrectomy was indicated in stages B4 and B5. The criteria for exclusion were previous intraocular surgery, a history of ocular inflammation, retinal detachment associated with a retinal tear, age >80 years, renal and hematologic diseases, uremia, prior chemotherapy, and the presence of chronic pathologies other than diabetes.
All of our patients underwent a vitrectomy with a lensectomy without IOL implantation for the treatment of severe PDR; an IOL was implanted only after confirming that the retinopathy activity had decreased. The IOL implantation was performed at least 90 days after the final vitrectomy. The inclusion criteria for performing a secondary IOL implantation were IOP<20 mm Hg and no apparent intraocular inflammation, rubeosis iridis, vitreous hemorrhage, or proliferative tissue. All patients underwent a comprehensive ocular examination before treatment and periodically up to 6 months after the treatments. This study was approved by the Fukuoka University Clinical Research ethics committee. The procedures performed conformed to the tenets of the Declaration of Helsinki.
Sample collection
Vitreous samples were obtained at the time of the first PPV and secondary IOL implantation or second vitrectomy in patients with PDR. At the beginning of the first PPV, at least 0.5 ml of undiluted core vitreous samples was collected with a 20-gauge cutter probe before opening the infusion port. At the beginning of the second vitrectomy or secondary IOL implant, at least 0.5 ml of undiluted vitreous fluid was collected with a 27-gauge syringe. The samples were collected into sterile plastic tubes and transferred to the laboratory immediately on ice. The sample was centrifuged for 10 min at 4 °C at 3000 r.p.m. (1630 × g). The supernatant was aliquoted and stored at –70 °C until measured. During the vitrectomy, we delaminated the fibrovascular proliferative membranes, removed the posterior vitreous around the macula, and performed panretinal endolaser photocoagulation of the retina up to the ora serrata. If retinal detachment was detected or developed, it was treated with air tamponade. At the end of the vitreous surgery, an ~6-mm diameter hole was made at the center of the anterior capsule, resulting in communication between the anterior chamber and vitreous cavity. This enabled us to obtain vitreous fluids from the anterior vitreous cavity at the beginning of the second surgery.
Measurement of vitreous cytokine levels
The VEGF and MCP-1 levels in vitreous samples were determined using commercial sandwich enzyme-linked immunosorbent assay kits from R&D Systems (Minneapolis, MN, USA). The VEGF kit detected the two short, secreted VEGF isoforms (VEGF121 and VEGF165), but not the two longer cell-associated isoforms. Each assay was performed according to the manufacturer’s protocol. The plates were incubated with 100 μl of VEGF or MCP-1 standards and diluted vitreous samples. The optical density was determined at 450 nm with the wavelength corrected at 540 nm using a microplate reader (Biotec, Tokyo, Japan). The minimum detectable concentrations of VEGF and MCP-1 were 15.6 and 31.2 pg/ml, respectively (the intra-assay coefficient of variation (CV) was 4.7% and the inter-assay CV was 6.7% for VEGF vs 4.7 and 5.8%, respectively, for MCP-1).
Statistics
Statistical analyses consisted of the Mann–Whitney’s U-test and Spearman’s rank correlation coefficient (correlation between proteins and duration) as appropriate using the program SPSS Science (SPSS, Chicago, IL, USA). All the data are presented as the mean±standard deviation. Boxplot diagrams were used to show the median value and quartiles.
Results
The second surgery cases
Twenty-one eyes of 21 patients (12 men, 9 women) required a second surgery because of prolonged or severe diabetic retinopathy activity. Their mean age was 56.4±10.0 years (range, 37–72 years), and the mean duration of diabetes was 13.5±7.7 years (range 1–28 years). All of the eyes were diagnosed with PDR. The mean HbA1c was 7.5±1.7% (range, 5.6–10.9%). The reasons for the second surgery were TRD (six eyes), NVG (three eyes), PVH (three eyes), macular pucker (one eye), and secondary IOL implant (seven eyes). One patient had both NVG and TRD. The basic features of these patients are summarized in Table 1.
PPV changed the intravitreal VEGF/MCP-1 concentrations after vitrectomy
The VEGF level did not decrease at the time of the second surgery (1.09±1.51 ng/ml) compared with the first vitrectomy (0.81±0.88 ng/ml, NS, n=21), whereas the MCP-1 level increased significantly at the time of the second surgery (2.20±2.21 ng/ml) compared with the first (0.72±0.57 ng/ml, P<0.01, n=21; Figure 1).
The concentration of VEGF/MCP-1 changes after vitrectomy. (a) The VEGF level did not decrease at the time of the second surgery (1.09±1.51 ng/ml) compared with the first vitrectomy (0.81±0.88 ng/ml, NS, n=21). (b) The MCP-1 level increased significantly at the time of the second surgery (2.20±2.21 ng/ml) compared with the first (0.72±0.57 ng/ml, P=0.003, n=21). The horizontal lines indicate the change in levels for individual patients between the first and second operations.
To investigate the relationship between VEGF/MCP-1 and the interval after the first vitrectomy, a correlation coefficient was calculated using Spearman’s rank correlation coefficient. Neither the VEGF nor the MCP-1 levels after the first surgery were correlated with the interval after the first surgery (VEGF: P=–0.06; MCP-1: P=–0.26; Figure 2). However, the MCP-1 level after the first surgery tended to increase immediately and then decrease with time. We set the mean±SD of the MCP-1 concentration at the first surgery as the baseline level. Within 60 days of the surgery, none of five patients had a MCP-1 concentration below baseline (mean±SD, 1.29 ng/ml) at the first surgery, whereas 9 of 16 patients (56.3%) had a MCP-1 concentration below the mean±SD after 60 days postoperatively. In the secondary IOL implant cases, 6 of 7 patients (85.7%) had MCP-1 concentrations below the mean±SD 90 days after surgery.
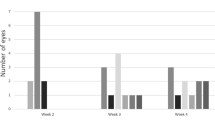
The kinetics of VEGF and MCP-1 after the first vitrectomy. Bold horizontal broken lines indicat the mean levels and fine broken lines indicat the mean+SD levels of VEGF/MCP-1 at first surgery. Neither the VEGF nor the MCP-1 levels after the first surgery were correlated with the interval after the first surgery (VEGF: P=–0.06, MCP-1: P=–0.26; Figure 2). However, the MCP-1 level after the first surgery tended to increase immediately and then decrease with time.
The VEGF/MCP-1 levels after vitrectomy (second vitrectomy vs secondary IOL implant)
At the first vitrectomy, comparison of the second vitrectomy cases and secondary IOL implants showed no significant differences in the concentrations of both VEGF and MCP-1 (second vitrectomy: VEGF 0.99±1.03 ng/ml, MCP-1 0.72±0.58 ng/ml; secondary IOL implant: VEGF 0.49±0.46 ng/ml, MCP-1 0.73±0.60 ng/ml; Figure 3).
The VEGF and MCP-1 levels after vitrectomy (the second vitrectomy vs the secondary IOL implant). (a) The VEGF concentrations in the second vitrectomy cases did not decrease at the time of the second vitrectomy compared with the first (1.58±1.64 vs 0.99±1.03 ng/ml, NS, n=14), whereas they decreased significantly at the time of the secondary IOL implant compared with the first vitrectomy (0.10±0.17 vs 0.49±0.46 ng/ml, P=0.05, n=7). (b) The vitreous MCP-1 level was significantly elevated at the second vitrectomy compared with the first surgery (2.87±2.46 vs 0.72±0.58 ng/ml, P<0.01, n=14), whereas it did not change at the time of the secondary IOL implant compared with the first vitrectomy (0.88±0.40 vs 0.73±0.60 ng/ml, NS, n=7). There were no significant differences in the protein values between IOL implant and secondary vitrectomy samples at first operation. The horizontal lines indicate the change in levels for individual patients between the first and second operations.
The VEGF concentrations in the second vitrectomy cases did not decrease at the time of the second vitrectomy compared with the first (1.58±1.64 vs 0.99±1.03 ng/ml, NS, n=14), whereas they decreased significantly at the time of the secondary IOL implant compared with the first vitrectomy (0.10±0.17 vs 0.49±0.46 ng/ml, P=0.05, n=7; Figure 3a). The vitreous MCP-1 level was significantly elevated at the second vitrectomy compared with the first surgery (2.87±2.46 vs 0.72±0.58 ng/ml, P<0.01, n=14), whereas it did not change at the time of the secondary IOL implant compared with the first vitrectomy (0.88±0.40 vs 0.73±0.60 ng/ml, NS, n=7; Figure 3b).
The causal relationships between the VEGF/MCP-1 levels and the reasons for the second vitrectomy
Next, we addressed the causal relationships between the MCP-1/VEGF levels and the reasons for the second surgery.
Tractional retinal detachment
We experienced seven eyes (including one patient with NVG+TRD) with TRD for durations ranging from 12 to 169 days after the first vitrectomy. In the case with the shortest interval, the MCP-1 level increased markedly at a concentration of 8.00 ng/ml, which was more than ten times higher than the mean value of the MCP-1 at the first vitrectomy. In contrast, the VEGF level of that patient was below measurable limits (Figure 4). The MCP-1 levels in patients with TRD at the second vitrectomy were significantly elevated compared with those with other reasons including NVG, PVH, macular pucker, and secondary IOL implant (TRD, n=7: 3.18±2.27 ng/ml; other reasons, n=14: 1.72±2.10 ng/ml; P<0.02, Mann–Whitney’s U-test). The VEGF level did not significantly change (TRD, n=7: 1.30±1.35 ng/ml; other reasons, n=14: 0.98±1.61 ng/ml, NS, Mann–Whitney’s U-test). Because the MCP-1 level tended to increase immediately after the surgery and it essentially returned to the baseline level 60 days after the first vitrectomy, we compared the MCP-1 level of the second vitrectomy cases with TRD with those for other reasons 60 days after the first surgery. The vitreous level of MCP-1 with TRD was 2.38±0.99 ng/ml, which was significantly elevated compared with those with other reasons (0.83±0.46 ng/ml; P<0.01, TRD n=5, other reasons n=11; Figure 4b). Three of five TRD cases had extremely high MCP-1 levels (>1.86 ng/ml; greater than the mean+2 SD at the first surgery) even 4 months after the first vitrectomy and these three patients had anterior hyaloid fibrovascular proliferation (AHFVP) at the second vitrectomy (Figure 4b).
The causal relationships between the either VEGF or MCP-1 levels at second surgery and the reasons for the second vitrectomy. Bold horizontal broken lines indicated the mean levels and fine broken lines indicated the mean+SD levels of VEGF or MCP-1 at first surgery. TRD, Tractional retinal detachment; NVG, neovascular glaucoma; PVH, persistent vitreous hemorrhage.
Neovascular glaucoma
We experienced four eyes (including one NVG+TRD case) with NVG and the VEGF levels were significantly elevated in all four cases compared with those with other reasons including TRD, PVH, macular pucker, and secondary IOL implant (NVG n=4: 3.33±1.67 ng/ml; other reasons n=17: 0.56±0.87 ng/ml, P<0.01, Mann–Whitney’s U-test; Figure 4a). In three of the four NVG cases, the second vitrectomy was performed 2 months after the first. In the other case, the second vitrectomy was performed 5 months after the first surgery and this patient had both TRD and NVG. The MCP-1 levels of the NVG cases were not significantly different compared with other reasons (NVG n=4: 1.05±0.85 ng/ml; other reasons n=17: 2.48±2.36 ng/ml, P>0.10, Mann–Whitney’s U-test). Next, we compared the MCP-1 level in NVG with other reasons 60 days after the first surgery. The MCP-1 concentration in NVG was 1.05±0.85 ng/ml, which was not significantly different compared with other reasons (1.40±1.03 ng/ml, NS, NVG n=4, other reasons n=12; Figure 4b).
Persistent vitreous hemorrhage
We experienced three eyes with PVH, and the VEGF level differed in each. As the second vitrectomy was performed within 3 weeks in all PVH cases, the MCP-1 level was extremely high (PVH n=3: 4.96±2.72 ng/ml). The mean MCP-1 level in these cases was more than six times higher than the mean MCP-1 at the first vitrectomy (Figure 4b).
We had only one eye with macular pucker in our cases, and the concentrations of both VEGF and MCP-1 were low in this case.
Discussion
We found that the MCP-1 concentration was elevated in the vitreous of patients with PDR after the vitreous surgery. The MCP-1 level tended to increase immediately after the surgery and then decrease with time. We also found that the MCP-1 level in the second surgery cases with TRD was significantly higher than in those with other reasons (PVH, NVG, macular pucker, and secondary IOL implant).
MCP-1 is induced by tissue injury, infection, and inflammation.6, 7 Therefore, the MCP-1 level being elevated immediately after the surgery was expected. In our series, the MCP-1 level in patients with PDR had essentially returned to the pre-operative level 60 days after the first vitrectomy. The secondary IOL implant group might represent the kinetics of MCP-1 levels in successful surgical treatment of PDR. In this group, the IOL implant was postponed at the first vitrectomy due to the severity of the diabetic retinopathy. After the diabetic retinopathy entered remission, the vitreous sample was collected at the beginning of IOL implantation. The shortest interval to the secondary IOL implantation was 3 months. The MCP-1 levels in all cases with complications except macular pucker were the same as or higher than in all IOL implant cases. This suggests that prolonged inflammation existed in the vitreous cavity at the second vitrectomy. In addition, patients who needed reoperation because of TRD had particularly high MCP-1 levels at all periods examined. Although previous reports have already revealed that the MCP-1 level in the vitreous with proliferative vitreoretinopathy, retinal detachment, and PDR was significantly higher compared with that in idiopathic epiretinal membrane,14,15,16 this is the first report showing that the MCP-1 level in TRD after vitreous surgery was significantly elevated. In Figure 4b, bold horizontal broken lines and fine broken lines indicated the mean and mean+SD levels of MCP-1 at first surgery, respectively. Three of five patients with TRD had extremely high MCP-1 levels (>1.86 ng/ml; greater than the mean+2 SD at the first surgery) even 4 months after the first vitrectomy, and these three patients had AHFVP at the second vitrectomy. Although the sample size was small in this study, the data suggest an association between MCP-1 and TRD after a vitrectomy, including AHFVP. Because signaling by MCP-1/CCR-2, an MCP-1 receptor, has already been proven to promote pulmonary,17 kidney,18 and liver fibrosis,19 further study with a large sample size is needed to elucidate the contribution of MCP-1 to repeat proliferative reactions in the eye.
The VEGF signal protein produced by cells stimulates ocular angiogenesis in PDR.1 Unlike MCP-1, the VEGF level did not rise after vitrectomy. In Figure 4a, bold horizontal broken lines indicat the mean levels of VEGF at first surgery. Within 3 weeks of the first operation, the VEGF level in three of four patients (75%) fell below the mean of the first vitreous surgery. Several reports have shown that the VEGF level was elevated in patients with NVG.20, 21, 22 We confirmed that the VEGF level in NVG was extremely high compared with those in other cases. Conversely, three of four (75%) patients with NVG at the second vitrectomy had MCP-1 levels similar to those at the first surgery. In one high MCP-1 case (>1.86 ng/ml; greater than the mean+2SD at the first surgery), AHFVP coexisting with NVG was observed at the second surgery. From these data, MCP-1, unlike VEGF, might have a minor role in promoting NVG.
As mentioned in the ‘Patients and methods’ section, a hole in the center of anterior capsule was made at the end of the first surgery, and this procedure is not routine in vitrectomy surgery. Lens epithelial cells exist on the anterior capsule and these cells sometimes make thick opacity in spite of scrubbing the anterior capsule by a cutter. To prevent thick opacity formation, the hole in the center of the anterior capsule was basically created. This procedure incidentally enabled us to collect the vitreous samples at the second surgery. To be exact, a communication between the vitreous and anterior chambers might alter the concentrations of VEGF and MCP-1 in the vitreous even though the aqueous humor is physiologically secreted from the vitreous side.

References
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994; 331: 1480–1487.
Oshima Y, Shima C, Wakabayashi T . Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology 2009; 116: 927–938.
Schiff WM, Barile GR, Hwang JC, Tseng JJ, Cekiç O, Del Priore LV et al. Diabetic vitrectomy: influence of lens status upon anatomic and visual outcomes. Ophthalmology 2007; 114: 544–550.
Eliott D, Lee MS, Abrams GW . Proliferative diabetic retinopathy: principles and techniques of surgicaltreatment. Retina, 4th edn. Elsevier Mosby: Philadelphia, PA, USA, 2006.
Yorston D, Wickham L, Benson S, Bunce C, Sheard R, Charteris D et al. Predictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathy. Br J Ophthalmol 2008; 92: 365–368.
Carr MW, Roth SJ, Luther E, Rose SS, Springer TA . Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA 1994; 91: 3652–3656.
Xu LL, Warren MK, Rose WL, Gong W, Gong W, Wang JM . Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukoc Biol 1996; 60: 365–371.
Yoshida S, Yoshida A, Ishibashi T . Induction of IL-8, MCP-1, and bFGF by TNF-alpha in retinal glial cells: implications for retinal neovascularization during post-ischemic inflammation. Graefes Arch Clin Exp Ophthalmol 2004; 242: 409–413.
Ivacko J, Szaflarski J, Malinak C, Flory C, Warren JS, Silverstein FS et al. Hypoxic-ischemic injury induces monocyte chemoattractant protein-1 expression in neonatal rat brain. J Cereb Blood Flow Metab 1997; 17: 759–770.
Angele MK, Knöferl MW, Ayala A, Albina JE, Cioffi WG, Bland KI et al. Trauma-hemorrhage delays wound healing potentially by increasing pro-inflammatory cytokines at the wound site. Surgery 1999; 126: 279–285.
Yoshida A, Elner SG, Bian ZM, Kunkel SL, Lukacs NW, Elner VM et al. Thrombin regulates chemokine induction during human retinal pigment epithelial cell/monocyte interaction. Am J Pathol 2001; 159: 1171–1180.
Yoshimura T, Sonoda KH, Sugahara M, Mochizuki Y, Enaida H, Oshima Y et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One 2009; 4: e8158.
Fukuda M . Classification and treatment of diabetic retinopathy. Diabetes Res Clin Pract 1994; 24: S171–S176.
Abu el-Asrar AM, Van Damme J, Put W, Veckeneer M, Dralands L, Billiau A et al. Monocyte chemotactic protein-1 in proliferative vitreoretinal disorders. Am J Ophthalmol 1997; 123: 599–606.
Elner SG, Elner VM, Jaffe GJ, Stuart A, Kunkel SL, Strieter RM et al. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res 1995; 14: 1045–1053.
Capeans C, De Rojas MV, Lojo S, Salorio MS . C-C chemokines in the vitreous of patients with proliferative vitreoretinopathy and proliferative diabetic retinopathy. Retina 1998; 18: 546–550.
Moore BB, Paine R III, Christensen PJ, Moore TA, Sitterding S, Ngan R et al. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol 2001; 167: 4368–4377.
Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M et al. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol 2004; 165: 237–246.
Mitchell C, Couton D, Couty JP, Anson M, Crain AM, Bizet V et al. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol 2009; 174: 1766–1775.
Itakura H, Kishi S, Kotajima N, Murakami M . Persistent secretion of vascular endothelial growth factor into the vitreous cavity in proliferative diabetic retinopathy after vitrectomy. Ophthalmology 2004; 111: 1880–1884.
Sone H, Okuda Y, Kawakami Y, Hanatani M, Suzuki H, Kozawa T et al. Vascular endothelial growth factor level in aqueous humor of diabetic patients with rubeotic glaucoma is markedly elevated. Diabetes Care 1996; 19: 1306–1307.
Tripathi RC, Li J, Tripathi BJ, Chalam KV, Chalam KV, Adamis AP . Increased level of vascular endothelial growth factor in aqueous humor of patients with neovascular glaucoma. Ophthalmology 1998; 105: 232–237.
Acknowledgements
This study was financially supported by Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science (Grant #24592687 and 23592574). The English language in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/kDc3GK.
Author contributions
Research planning was done by SY. Collecting of samples was done by YS, SY, and TK. Measuring the MCP-1/VEGF levels was done by SY. Data management in order to protect privacy was done by KI and RA. Data analysis was performed by YS. Funds were procured by YS, SY, and TI. YS wrote the manuscript. This study was supervised by TK and TI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sassa, Y., Yoshida, S., Ishikawa, K. et al. The kinetics of VEGF and MCP-1 in the second vitrectomy cases with proliferative diabetic retinopathy. Eye 30, 746–753 (2016). https://doi.org/10.1038/eye.2016.20
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.20
This article is cited by
-
Changes in aqueous and vitreous inflammatory cytokine levels in proliferative diabetic retinopathy: a systematic review and meta-analysis
Eye (2022)
-
Systemic and vitreous biomarkers — new insights in diabetic retinopathy
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)
-
Cytokines associated with hemorrhage in proliferative diabetic retinopathy
International Ophthalmology (2021)
-
Monocyte chemoattractant protein-1 (MCP-1/CCL2) in diabetic retinopathy: latest evidence and clinical considerations
Journal of Cell Communication and Signaling (2019)