Abstract
Background
Diabetic retinopathy is a major complication of diabetes mellitus, where in its most advanced form ischemic changes lead to the development of retinal neovascularization, termed proliferative diabetic retinopathy (PDR). While the development of PDR is often associated with angiogenic and inflammatory cytokines, studies differ on which cytokines are implicated in disease pathogenesis and on the strength of these associations. We therefore conducted a systematic review and meta-analysis to quantitatively assess the existing body of data on intraocular cytokines as biomarkers in PDR.
Methods
A comprehensive search of the literature without year limitation was conducted to January 18, 2021, which identified 341 studies assessing vitreous or aqueous cytokine levels in PDR, accounting for 10379 eyes with PDR and 6269 eyes from healthy controls. Effect sizes were calculated as standardized mean differences (SMD) of cytokine concentrations between PDR and control patients.
Results
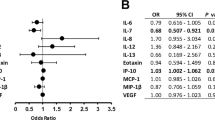
Concentrations (SMD, 95% confidence interval, and p-value) of aqueous IL-1β, IL-6, IL-8, MCP-1, TNF-α, and VEGF, and vitreous IL-2, IL-4, IL-6, IL-8, angiopoietin-2, eotaxin, erythropoietin, GM-CSF, GRO, HMGB-1, IFN-γ, IGF, IP-10, MCP-1, MIP-1, MMP-9, PDGF-AA, PlGF, sCD40L, SDF-1, sICAM-1, sVEGFR, TIMP, TNF-α, and VEGF were significantly higher in patients with PDR when compared to healthy nondiabetic controls. For all other cytokines no differences, failed sensitivity analyses or insufficient data were found.
Conclusions
This extensive list of cytokines speaks to the complexity of PDR pathogenesis, and informs future investigations into disease pathogenesis, prognosis, and management.
摘要
糖尿病视网膜病变是糖尿病的主要并发症, 其晚期的缺血性改变可导致视网膜新生血管的形成, 称为增殖性糖尿病视网膜病变(PDR)。虽然PDR的发展通常与血管生成和炎性细胞因子有关, 但对于哪些细胞因子与发病机制有关以及这些因子的关联强度, 目前的研究结果仍存在分歧。因此, 我们进行了系统的综述和荟萃分析, 以定量评估现有的眼内细胞因子作为PDR生物标记物的数据。
本文进行了全面检索, 截止至2021年1月18日, 共纳入341篇探究增生性视网膜病变玻璃体或房水细胞因子水平的研究, 其中10379只眼为增殖性视网膜病变, 6269只眼来自健康对照组。效应大小以PDR患者和对照组患者之间细胞因子浓度的标准化平均差(SMD)计算。
与健康对照组相比, PDR患者的细胞因子显著升高(SMD、95%可信区间和p值), 包括房水中的IL-1β、IL-6、IL-8、MCP-1、TNF-α和VEGF, 以及玻璃体中IL-2、IL-4、IL-6、IL-8、血管生成素-2、嗜酸性粒细胞趋化因子、促红细胞生成素、GM-CSF、GRO、HMGB-1、IFN-γ、IGF、IP-10、MCP-1、MIP-1、MMP-9、PDGF-AA、PlGF、sCD40L、SDF-1、sICAM-1、sVEGF-1、sVEGFR、TIMP、TNF-1和VEGF。其他细胞因子未发现显著性差异、敏感性分析异常或数据丢失。
这一广泛的细胞因子结果说明了PDR发病机制的复杂性, 并对今后疾病的发病机制、预后和治疗的研究提供了信息。
Similar content being viewed by others
introduction
Proliferative diabetic retinopathy (PDR) represents the most advanced stage of diabetic retinopathy. PDR affects approximately 17 million people globally and is a leading cause of blindness among adults [1], making it a significant global health and economic problem. Characterized by neovascularization at the interface of perfused and non-perfused tissue, fragile new vessel formation and progressive fibrosis places patients with PDR at risk of severe visual compromise from vitreous haemorrhage, tractional retinal detachment, and neovascular glaucoma. While the pathophysiology of PDR is complex and not yet completely understood, it is known that microvascular ischemia, chronic inflammation, and retinal neurodegeneration are all important in the disease process [2].
As a result of prolonged hyperglycaemia, retinal blood vessels undergo pericyte and endothelial cell apoptosis [3, 4]. The resulting capillary occlusion and ischemia leads to upregulation of hypoxia-inducible factor 1 and angiogenic factors, the most studied of which is vascular endothelial growth factor (VEGF). VEGF contributes to the progression of PDR through increased vascular permeability and the promotion of endothelial cell proliferation [2]. The management of PDR with anti-VEGF therapy, therefore, has become increasingly common, although pan-retinal photocoagulation remains the mainstay of treatment. However, some patients may progress despite available therapies, including anti-VEGF agents and there remains a need for additional therapeutic targets and improved treatment algorithms.
The search for other intraocular cytokines that are involved in PDR pathogenesis has yielded a large body of literature. In addition to VEGF, angiogenic factors including insulin-like growth factor-I, basic fibroblast growth factor, platelet derived growth factor, placental growth factor, and angiopoietin have all been implicated in retinal neovascularization [5, 6]. However, there remains inconsistency between studies regarding which cytokines are associated with PDR and the magnitude of this association. Clarity on the role of cytokines in PDR will aid in predicting disease severity, progression, treatment response, and identifying novel therapeutic targets. We have therefore conducted a systematic review and meta-analysis on the association of intraocular cytokines and PDR to address these inconsistencies and to quantitatively summarize the literature.
Methods
A detailed account of the methods used in this meta-analysis is available in our companion article on nonproliferative diabetic retinopathy (NPDR) in this issue, and is similar to those used in our previous work on a diabetic macular oedema [7]. In brief, a systematic literature search was done using Ovid MEDLINE, Embase, and Web of Science databases without year limitation until January 18, 2021. The search statements are available in Supplementary Fig. S1.
Studies were assessed by two independent reviewers using the following exclusion criteria: (1) the study did not examine a correlation, clinical outcome or response to treatment of an aqueous or vitreous cytokine; (2) was on subjects other than human adults; (3) included subjects with diabetic macular oedema; and (4) was a review article, editorial, or opinion piece. Studies that assessed the correlation of cytokines to clinical outcomes (prognostic biomarkers) or disease correlation to cytokine concentrations (diagnostic biomarkers) were evaluated for their risk of bias using the Quality in Prognosis Studies (QUIPS) tool or the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool, respectively [8, 9]. Modifications to QUIPS and QUADAS are provided in Supplementary Fig. S2.
Primary outcome measures were mean and standard deviation intraocular cytokine concentration and p-values for comparisons between those with PDR and non-diabetic participants, if a control group was available. Review Manager (Version 5.3.5, Nordic Cochrane Centre, Copenhagen, Denmark) was used to compute standardized mean difference (SMD) and 95% confidence interval for cytokine concentrations between patients with PDR and controls using the inverse variance method when there was sufficient data of at least three data points. The formulation of the SMD used was Hedges’ adjusted g, selected as it includes an adjustment for small sample bias. A random-effects model was chosen because within-study and between-study variances were hypothesized to influence the true effect size. The magnitude of the SMD was defined as being very small if <0.20, small if 0.20-0.49, medium if 0.50–0.79, and large if ≥0.80. Between-study heterogeneity was assessed using the Cochrane Q test and I2 statistic as described previously. Statistical difference for the Cochrane Q test was set as p < 0.05; I2 statistics of 0.25, 0.50 and 0.75 denoted low, medium and high levels of heterogeneity, respectively, as per previous literature [10].
A sensitivity analysis was undertaken by removing one study at a time to assess outcome stability; if the effect size significantly changed with the removal of a single study, the data for that cytokine was deemed to have failed the sensitivity analysis. To determine if the results of this study were influenced by participants having previously received treatment for their PDR, two subgroup analyses were performed: one included only studies where patients had received no treatments for PDR within 3 months of sample collection; the other included only studies where all patients were treatment naïve.
Results
The initial search identified 2947 records, of which 1681 remained after automated removal of duplicates. Following review at the full-text level to ensure that no cytokine concentrations were missed, 480 studies met the inclusion/exclusion criteria and 341 of those were specific for PDR (Fig. 1). These 341 studies encompassed for 10379 eyes with PDR and 6269 eyes from healthy controls [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350,351].
Study characteristics
Population and study characteristics are summarized in Table 1. Vitreous cytokines were assessed in 82% (279/341) of studies, with only 12% (41/341) assessing aqueous cytokines and the remaining 6% (21/341) assessing both vitreous and aqueous cytokines. Most studies (57%, 193/341) did not specify the type of diabetes in PDR patients, while 24% (81/341) of studies used participants with type 2 diabetes, 1% (3/341) of studies used those with type 1, and 14% (46/341) used patients with either type 1 or type 2 diabetes. The remaining studies classified patients by treatment status; 0.6% (2/341) were insulin dependent, 0.3% (1/341) were non-insulin dependent, and 4% (14/341) used both insulin dependent and independent patients. The analytical method used to quantify cytokine concentrations was clearly stated in 99% (340/341) studies, with the enzyme-linked immunosorbent assay and multiplex assay being most frequently used in 70% (239/341). Of the 293 studies with a control arm, the most commonly used control was eyes with epiretinal membrane or macular hole (38%, 111/293), with cataract surgery, retinal detachment, macular pucker, vitreous haemorrhage, vitreous floaters, vitreomacular traction, lens dislocation, and lens subluxation also being used as controls.
Quality assessment
Diagnostic biomarker studies
Two hundred and seventy-nine studies (82%) evaluated the diagnostic potential of intraocular cytokines and were assessed for quality using QUADAS (Fig. 2a), with further details available for individual studies in Supplemental Table S1. A case-control design was used in 88% (246/279) of studies and the selection criteria were clearly stated in 71% (198/279). There was a low risk of selection bias in 70% (195/279). Selected patients were an appropriate match for the study question in every case and all enrolled patients were included in the final analysis in 94% (263/279) of studies. The index text demonstrated a low risk of study bias in all studies, but was never interpreted without knowledge of the reference standard. All studies had each participant receiving the same reference standard, classified PDR appropriately and matched the review question, and in no studies was it interpreted independently from the index test (100%, 279/279). There was an overall low risk of bias in diagnostic studies.
Prognostic biomarker studies
Sixty-two studies (18%) evaluated the prognostic potential of cytokines. The overall QUIPS assessment is shown in Fig. 2b, and study details are available in Supplemental Table S2. There was a low risk of bias in study participation in 71% (44/62), and study attrition had a low risk of bias in 85% (53/62) of studies. Data collection and outcome measurement, respectively, had a low risk of bias in 81% (50/62) and 100% (62/62) of studies. An unclear risk of bias in confounding was found in most studies (85%; 53/62) as most studies did not account for non-diabetic ocular or systemic conditions that may influence intraocular cytokine concentrations. Finally, 81% (50/62) of studies had an appropriate statistical analysis. Prognostic studies were found to have an overall low risk of bias.
Main association of DME with cytokine levels
Fifty-four cytokines had sufficient data for inclusion in the meta-analysis, with results detailed in Table 2. Aqueous concentrations (standard mean difference, 95% confidence interval, and p value) of IL-1β (5.76, 2.45–9.07, p = 0.0006), IL-6 (2.69, 1.34–4.03, p < 0.0001), IL-8 (2.77, 1.11–4.43, p = 0.001), MCP-1 (1.80, 0.72–2.88, p = 0.001), TNF-α (9.74, 5.01 to 14.47, p < 0.0001), and VEGF (2.31, 1.61–3.00, p < 0.00001) were significantly higher in patients with PDR when compared to healthy nondiabetic controls. For vitreous cytokines, concentrations of IL-2 (0.61, 0.18 to 1.04, p = 0.006), IL-4 (1.07, 0.30–1.84, p = 0.007), IL-6 (1.72, 1.30–2.13, p < 0.00001), IL-8 (1.78, 1.16–2.39, p < 0.00001), angiopoietin-2 (1.46, 0.93–1.98, p < 0.00001), eotaxin (0.99, 0.36–1.63, p = 0.002), erythropoietin (1.37, 0.81–1.93, p < 0.00001), GM-CSF (0.72, 0.34–1.10, p = 0.0002), GRO (1.40, 0.49–2.32, p = 0.003), HMGB-1 (0.86, 0.62–1.10, p < 0.00001), IFN-γ (0.75, 0.33–1.17, p = 0.0004), IGF (0.48, 0.17–0.80, p = 0.003), IP-10 (1.53, 0.87–2.20, P < 0.00001), MCP-1 (2.53, 1.76–3.30, p < 0.00001), MIP-1 (1.09, 0.28–1.91, p = 0.009), MMP-9 (0.92, 0.32–1.52, p = 0.003), PDGF-AA (1.28, 0.77–1.78, p < 0.00001), PlGF (1.33, 0.80–1.87, p < 0.00001), sCD40L (1.15, 0.30–2.01, p = 0.008), SDF-1 (1.60, 0.79–2.41, p = 0.0001), sICAM-1 (1.40, 0.73–2.07, p < 0.0001), sVEGFR (2.40, 0.92–3.89, p = 0.002), TIMP (0.73, 0.34–1.13, p = 0.0003), TNF-α (1.05, 0.34–1.77, p = 0.004), and VEGF (1.96, 1.69–2.23, p < 0.00001) were significantly elevated in subjects with PDR as compared to healthy controls. For all other cytokines intraocular concentrations were not significantly different between PDR and controls, did not pass the sensitivity analysis, or had insufficient data for inclusion in the meta-analysis.
Supplementary Table S3 shows the known associations of intraocular cytokines with PDR from the previous studies identified in the systematic review and contrasts those with the results of this meta-analysis. Of the 31 aqueous and vitreous cytokines for which we found a significant elevation in cytokine concentration in PDR versus controls, previous studies had found them to be either not significantly different or even reduced in PDR versus controls in 17% (120/700) of the cases. Forest plots for each of the analysed cytokines are available in Supplementary Fig. S3, and funnel plots for each cytokine that had at least five data points are available in Supplementary Fig. S4.
Some studies included patients that had received previous treatment for PDR, such as a laser photocoagulation or intravitreal anti-VEGF. To determine if the results of the meta-analysis was influenced by patient treatment status, two subgroup analyses were performed (Supplementary Fig. S5). When including only studies with patients that had no treatments for at least 3 months prior to study enrolment, the effect sizes for aqueous cytokines was 5.76 for IL-1β (2.45–9.07, p = 0.0006), 3.56 for IL-6 (2.03 to 5.09, p < 0.00001), 5.58 for IL-8 (2.97 to 8.19, p < 0.0001), 2.40 for MCP-1 (0.68–4.12, p = 0.006), 9.74 for TNF-α (5.01 to 14.47, p < 0.0001), and 2.25 for VEGF (1.54–2.97, p < 0.00001). Similarly, for vitreous cytokines the effect size was 1.48 for IL-6 (0.75–2.21, p < 0.0001), 1.37 for IL-8 (0.25–2.48, p = 0.02), 1.16 for IL-10 (0.44–1.89, p = 0.002), 1.20 for IFN-γ (0.47–1.94, p < 0.0001), 3.29 for MCP-1 (0.87–5.72, p = 0.008), –2.29 for PEDF (–3.87 to –0.70, p = 0.005), 0.91 for TNF-α (0.36–1.47, p = 0.001), and 3.11 for VEGF (2.29 to 3.93).
When including only studies with patients that had no previous treatments the effect size for aqueous VEGF was 3.08 (2.25–3.90, p < 0.00001), vitreous PEDF was –2.89 (–4.41 to –1.37, p = 0.0002), and vitreous VEGF was 3.71 (2.90–4.51, p < 0.00001). All other cytokines failed the sensitivity analysis.
Discussion
Panretinal photocoagulation has been a longstanding treatment for PDR. However, this treatment can restrict the visual field, reduce visual acuity at night, and has the potential to worsen macular oedema [352, 353]. The efficacy of intravitreal ranibizumab, an anti-VEGF agent, was compared to panretinal photocoagulation in DRCR Network Protocol S, a randomized trial of 394 eyes. This study found that ranibizumab was non-inferior to panretinal photocoagulation in terms of mean change in visual acuity and the proportion of eyes without neovascularization [354]. In both groups, however, at two years more than 40% of participants had active neovascularization on fundus photography and 2–3% had developed neovascular glaucoma. This suggests that proinflammatory or proangiogenic cytokines in addition to VEGF are likely involved in the disease process and may therefore be appropriate treatment targets.
Since 1992 there have been 341 studies on the association of intraocular fluid cytokines with PDR, with the vast majority being published in the last decade. Interpretation of this wealth of data has been complicated by inconsistencies in which cytokines may be involved in disease pathogenesis, with some studies even finding no difference in VEGF concentrations between patient with PDR and controls (for example Nishiguchi et al. 2013 [218]; Semeraro et al. 2014 [257]). Previous meta-analyses were highly selective in the cytokines investigated or did not address PDR specifically and looked at a relatively small number of studies [355, 356]. We therefore attempted to undertake a more comprehensive systematic review and meta-analysis on intraocular cytokines in PDR. This work summarizes 10379 eyes with PDR and 6269 eyes from healthy controls.
Previous studies have identified a correlation between aqueous and vitreous cytokine concentrations in diabetic retinopathy [324], and one might expect similar cytokine profiles in the aqueous and vitreous samples. In the end stage of severe retinal ischemic disease proinflammatory and proangiogenic cytokines can migrate from the posterior to anterior segment of the eye and promote new vessel formation on the iris surface or anterior chamber angle, leading to neovascular glaucoma [161, 275]. Over eighty percent (279/341) of the included studies utilized vitreous humour samples alone, which is appropriate for investigating PDR pathogenesis due to the proximity between the vitreous and the retina. However, this resulted in many aqueous cytokines having insufficient data for inclusion in the meta-analysis. Given the relative ease of aqueous paracentesis, it would be beneficial for future studies to collect both aqueous and vitreous humour samples to further elucidate the relationship between posterior and anterior disease processes.
This study found significant elevation of aqueous IL-1β, IL-6, IL-8, IP-10, MCP-1, TNF-α, and VEGF, and vitreous IL-4, IL-6, IL-8, IL-12, angiopoietin-2, eotaxin, erythropoietin, GRO, HMGB-1, IFN-γ, IGF, IP-10, MCP-1, MIP-1, MMP-9, PDGF-AA, PlGF, sCD40L, SDF-1, sICAM-1, sVEGFR, TIMP, TNF-α, and VEGF. Most of the cytokines significantly associated with PDR had a medium (0.50–0.79) to large (≥0.80) effect size and none were sensitive to the result of any one study. Many of these molecules are known to have an important proinflammatory and proangiogenic role, and for some there may be a synergistic or antagonistic affects amongst cytokines. Angiopoietin-2 influences blood-retinal barrier stabilization and vessel remodelling through mediation of Tie-2 phosphorylation and is elevated in the retina of diabetic patients with chronic hyperglycaemia [357, 358]. IFN-γ is a key participant in inflammation in diabetic retinopathy through the breakdown of the blood-retina barrier and upregulation of other proangiogenic cytokines [359]. This also induces the release of IP-10, another CXC chemokine that in turn prevents neovascularization and inhibits IL-8-induced chemotaxis [86, 360]. PDGF is a ubiquitous growth factor produced in the retina by the retinal pigment epithelium, astrocytes, and ganglion cells, and directly contributes to neovascularization and fibrovascular proliferation in PDR [361, 362]. PlGF, a member of the VEGF family, is not required for physiologic angiogenesis but does play a role under pathological conditions where levels correlate with PDR activity [133]. This cytokine is a target of aflibercept and concentrations may also decrease following conbercept injection [350]. TNF-α and sCD40-L, which is a member of the TNF superfamily, promote angiogenesis and may induce the expression of VEGF in vivo [169, 363].
The number of elevated cytokines in PDR is much greater than that seen in previous work on retinal vein occlusion [364], neovascular age-related macular degeneration [365], and diabetic macular oedema [7], and in our companion article on cytokines in NPDR. This is reflective of the complexity of PDR pathogenesis and the advanced disease state, where dysregulation of several biochemical and molecular signalling pathways is driven by oxidative stresses [366]. In the NPDR meta-analysis it was found that aqueous IL-6, IL-17, and VEGF as well as vitreous VEGF were elevated when compared to controls, all with large effect sizes. Apart from IL-17, which failed the sensitivity analysis, similar results were obtained for PDR. In addition, aqueous IL-1β, IL-8, and TNF-α were elevated in NPDR versus controls but failed sensitivity analysis, making their significance inconclusive. Given that these cytokines were elevated in PDR, it suggests that the importance of their role in the nonproliferative form of DR may be better elucidated with additional studies.
When including only studies where all participants were without treatment for their PDR in the preceding 3 months, aqueous IL-1β, IL-6, IL-8, MCP-1, TNF-α, and VEGF and vitreous IL-6, IL-8, IL-10, IFN-γ, MCP-1, TNF-α, and VEGF were found to be significantly elevated and, in most cases, had larger effect size than found in the primary analysis. Furthermore, when using only data from treatment-naive patients only aqueous and vitreous VEGF was significantly elevated in PDR, with other cytokines having an insufficient number of studies or a failed sensitivity analysis. Due to notable heterogeneity in treatment type, duration, and the use of combination therapy, we were not able to stratify patients by the type of prior treatment. Our analysis was further limited as some studies did not provide sufficient detail on the nature of prior treatments. It would be interesting to investigate the influence of specific prior treatments on the cytokine milieu since these may have different mechanisms and durations of action, allowing for a better understanding of the post-treatment cytokine profile.
In both the treatment-free for 3 months and treatment naïve subgroups PEDF was significantly lower in PDR than in controls, with an effect size of -2.29 (-3.87 to -0.70) in those without treatment for 3 months and -2.89 (-4.41 to -1.37) for those without any prior treatments. PEDF can counteract VEFG-induced vascular permeability and inhibit retinal neovascularization [362, 367, 368], acting as an inhibitor of VEGF function through its action on the VEGF receptor [369]. It is disruptions to the balance of proangiogenic and antiangiogenic cytokines because of chronic hyperglycaemia in diabetes that leads to proliferative retinopathy and its sequelae [53, 370]. It therefore fits mechanistically that patients with more active neovascularization would have lower levels of PEDF. While angiogenic stimuli such as VEGF are required for PDR to occur, pathology can only develop when the ‘break’ of negative regulators such as PEDF fail [371].
Of the 31 aqueous and vitreous cytokines for which we found a significant elevation, 17% (120/700) of previous investigations had found them to either be not significantly different or reduced in PDR when compared to controls (Table S3). There are several possible reasons for this inconsistency in the literature. There is a variable nature to an individual’s cytokine profile. While there is little data available on longitudinal intraocular cytokine concentrations, work on plasma cytokines indicates that levels are sensitive to the duration of diabetes [372], acute episodes of hyperglycaemia [373], and systemic factors such as hypertension [374] and dyslipidaemia [375]. Furthermore, the ‘healthy’ control groups often contained patients of different ethnicity and with either systemic or ocular conditions such as macular hole or epiretinal membrane that may influence cytokine concentrations [376, 377]. As studies generally had small sample sizes, with a median of 27 cases and 20 controls, they may be particularly susceptible to these influences. Future work using large ocular fluid biobanks may provide a normative database that identify genetic, temporal, and comorbid variations in the cytokine profile, and upon which changes specifically due to PDR can be further elucidated. Over 70% of studies utilized enzyme linked immunosorbent assays for cytokine quantification, with more recent studies being predominantly based on multiplex analysis. There remains both intra-assay and inter-assay variability due to differences in sample collection, handling, and storage as well as the types of buffers and antibodies used. It is unclear to what extent these impact reported cytokine concentrations, and if a correction factor should be applied when comparing data from different assay types.
Conclusions
Previous studies have shown conflicting associations for most cytokines assessed in PDR. This meta-analysis demonstrated elevated aqueous concentrations of IL-1β, IL-6, IL-8, MCP-1, TNF-α, and VEGF, and vitreous concentrations of IL-2, IL-4, IL-6, IL-8, angiopoietin-2, eotaxin, erythropoietin, GM-CSF, GRO, HMGB-1, IFN-γ, IGF, IP-10, MCP-1, MIP-1, MMP-9, PDGF-AA, PlGF, sCD40L, SDF-1, sICAM-1, sVEGFR, TIMP, TNF-α, and VEGF in patients with PDR.
When assessing patients without recent treatment, levels of the anti-angiogenic cytokine PEDF were low. This work identifies a number of candidate cytokines other than VEGF that are implicated in PDR and adds clarity to the large body of literature. These findings suggest potential biomarkers of PDR development and severity and point to potential therapeutic targets.
Supplemental information is available at Eye’s website.
References
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19:1816.
Bek T. Diameter changes of retinal vessels in diabetic retinopathy. Curr Diab Rep. 2017;17:82.
Beltramo E, Porta M. Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr Med Chem. 2013;20:218–25.
Cai J, Boulton M. The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye (Lond). 2002;16:242–60.
Noma H, Yasuda K, Shimura M. Involvement of Cytokines in the Pathogenesis of Diabetic Macular Edema. Int J Mol Sci. 2001;22:3427.
Minaker SA, Mason RH, Lahaie Luna G, Farahvash A, Garg A, Bhambra N, et al. Changes in aqueous and vitreous inflammatory cytokine levels in diabetic macular oedema: a systematic review and meta-analysis. Acta Ophthalmol. 2001;99:134–55.
Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–6.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36.
Qin X-Y, Zhang S-P, Cao C, Loh YP, Cheng Y. Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease. JAMA Neurol. 2016;73:1316–9.
Abdel Rasol HA, Azab A. Vitreous, aqueous, and serum levels of vascular endothelial growth factor and angiopoietin-2 in patients with proliferative diabetic retinopathy and diabetic macular edema. Middle E J Ophthalmol. 2007;14:3–6.
Abu el Asrar AM, Maimone D, Morse PH, Gregory S, Reder AT. Cytokines in the vitreous of patients with proliferative diabetic retinopathy. Am J Ophthalmol. 1992;114:731–6.
Abu el-Asrar AM, Van Damme J, Put W, Veckeneer M, Dralands L, Billiau A, et al. Monocyte chemotactic protein-1 in proliferative vitreoretinal disorders. Am J Ophthalmol. 1997;123:599–606.
Abu El-Asrar AM, Struyf S, Kangave D, Geboes K, Van Damme J. Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur Cytokine Netw. 2006;17:155–65.
Abu El-Asrar AM, Nawaz MI, Kangave D, Geboes K, Ola MS, Ahmad S, et al. High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Mol Vis. 2011;17:1829–38.
Abu El-Asrar AM, Nawaz MI, Kangave D, Abouammoh M, Mohammad G. High-mobility group box-1 and endothelial cell angiogenic markers in the vitreous from patients with proliferative diabetic retinopathy. Mediators Inflamm. 2012;2012:697489.
Abu El-Asrar AM, Mohammad G, Nawaz MI, Siddiquei MM, Kangave D, Opdenakker G. Expression of lysophosphatidic acid, autotaxin and acylglycerol kinase as biomarkers in diabetic retinopathy. Acta Diabetol. 2013;50:363–71.
Abu El-Asrar AM, Mohammad G, Nawaz MI, Siddiquei MM, Van den Eynde K, Mousa A, et al. Relationship between vitreous levels of matrix metalloproteinases and vascular endothelial growth factor in proliferative diabetic retinopathy. PLoS One. 2013;8:e85857.
Abu El-Asrar AM, Nawaz MI, Kangave D, Mairaj Siddiquei M, Geboes K. Angiogenic and vasculogenic factors in the vitreous from patients with proliferative diabetic retinopathy. J Diabetes Res. 2013;2013:539658.
Abu El-Asrar AM, Nawaz MI, Kangave D, Siddiquei MM, Ola MS, Opdenakker G. Angiogenesis regulatory factors in the vitreous from patients with proliferative diabetic retinopathy. Acta Diabetol. 2013;50:545–51.
Abu El-Asrar AM, Nawaz MI, De Hertogh G, Alam K, Siddiquei MM, Van den Eynde K, et al. S100A4 is upregulated in proliferative diabetic retinopathy and correlates with markers of angiogenesis and fibrogenesis. Mol Vis. 2014;20:1209–24.
Abu El-Asrar AM, Alam K, Nawaz MI, Mohammad G, Van den Eynde K, Siddiquei MM, et al. Upregulated expression of heparanase in the vitreous of patients with proliferative diabetic retinopathy originates from activated endothelial cells and leukocytes. Invest Ophthalmol Vis Sci. 2015;56:8239–47.
Abu El-Asrar AM, De Hertogh G, Nawaz MI, Siddiquei MM, Van den Eynde K, Mohammad G, et al. The tumor necrosis factor superfamily members TWEAK, TNFSF15 and fibroblast growth factor-inducible protein 14 are upregulated in proliferative diabetic retinopathy. Ophthalmic Res. 2015;53:122–30.
Abu El-Asrar AM. Unregulated expression of heparanasein the vitreous of patients with proliferative diabetic retinopathy originates from activated endothelial cells and leukocytes. Ophthalmologica. 2016;236:37.
Abu El-Asrar AM, Alam K, Nawaz MI, Mohammad G, Van den Eynde K, Siddiquei MM, et al. Upregulation of thrombin/matrix metalloproteinase-1/protease-activated receptor-1 chain in proliferative diabetic retinopathy. Curr Eye Res. 2016;41:1590–1600.
Abu El-Asrar AM, Alam K, Garcia-Ramirez M, Ahmad A, Siddiquei MM, Mohammad G, et al. Association of HMGB1 with oxidative stress markers and regulators in PDR. Mol Vis. 2016;23:853–71.
Abu El-Asrar AM, Ahmad A, Alam K, Siddiquei MM, Mohammad G, Hertogh G, et al. Extracellular matrix metalloproteinase inducer (EMMPRIN) is a potential biomarker of angiogenesis in proliferative diabetic retinopathy. Acta Ophthalmol. 2017;95:697–704.
Abu El-Asrar AM, Ahmad A, Alam K, Bittoun E, Siddiquei MM, Mohammad G, et al. Unbalanced vitreous levels of osteoprotegerin, RANKL, RANK, and TRAIL in proliferative diabetic retinopathy. Ocul Immunol Inflamm. 2017;26:1248–60.
Abu El-Asrar AM, Struyf S, Mohammad G, Gouwy M, Rytinx P, Siddiquei MM, et al. Osteoprotegerin is a new regulator of inflammation and angiogenesis in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58:3189–201.
Abu El-Asrar AM, Ahmad A, Alam K, Bittoun E, Siddiquei MM, Mohammad G, et al. Association of 150-kDa oxygen-regulated protein with vascular endothelial growth factor in proliferative diabetic retinopathy. Acta Ophthalmol. 2018;96:e460–e467.
Abu El-Asrar AM, Mohammad G, Allegaert E, Ahmad A, Siddiquei MM, Alam K, et al. Matrix metalloproteinase-14 is a biomarker of angiogenic activity in proliferative diabetic retinopathy. Mol Vis. 2018;24:394–406.
Abu El-Asrar AM, Alam K, Siddiquei MM, Van den Eynde K, Mohammad G, De Hertogh G, et al. Myeloid-related protein-14/MRP-14/S100A9/Calgranulin B is associated with inflammation in proliferative diabetic retinopathy. Ocul Immunol Inflamm. 2018;26:615–24.
Abu El-Asrar AM, Ahmad A, Bittoun E, Siddiquei MM, Mohammad G, Mousa A, et al. Differential expression and localization of human tissue inhibitors of metalloproteinases in proliferative diabetic retinopathy. Acta Ophthalmol. 2018;96:e27–e37.
Abu El-Asrar AM, Ahmad A, Allegaert E, Siddiquei MM, Gikandi PW, De Hertogh G, et al. Interleukin-11 overexpression and M2 macrophage density are associated with angiogenic activity in proliferative diabetic retinopathy. Ocul Immunol Inflamm. 2019;28:575–88.
Abu El-Asrar AM, Ahmad A, Siddiquei MM, De Zutter A, Allegaert E, Gikandi PW, et al. The proinflammatory and proangiogenic macrophage migration inhibitory factor is a potential regulator in proliferative diabetic retinopathy. Front Immunol. 2019;10:2752.
Abu El-Asrar AM, Ahmad A, Allegaert E, Siddiquei MM, Alam K, Gikandi PW, et al. Galectin-1 studies in proliferative diabetic retinopathy. Acta Ophthalmol. 2020;98:e1–e12.
Adamiec-Mroczek J, Oficjalska-Młyńczak J. Assessment of selected adhesion molecule and proinflammatory cytokine levels in the vitreous body of patients with type 2 diabetes-role of the inflammatory-immune process in the pathogenesis of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246:1665–70.
Adamiec-Mroczek J, Oficjalska-Młyńczak J, Misiuk-Hojło M. Proliferative diabetic retinopathy - The influence of diabetes control on the activation of the intraocular molecule system. Diabetes Res Clin Pr. 2009;84:46–50.
Adamiec-Mroczek J, Oficjalska-Młyńczak J, Misiuk-Hojło M. Roles of endothelin-1 and selected proinflammatory cytokines in the pathogenesis of proliferative diabetic retinopathy: Analysis of vitreous samples. Cytokine. 2010;49:269–74.
Adamis AP, Miller JW, Bernal MT, D’Amico DJ, Folkman J, Yeo TK, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–50.
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7.
Ambati J, Chalam KV, Chawla DK, D’Angio CT, Guillet EG, Rose SJ, et al. Elevated gamma-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol. 1997;115:1161–6.
Arimura N, Otsuka H, Yamakiri K, Sonoda Y, Nakao S, Noda Y, et al. Vitreous mediators after intravitreal bevacizumab or triamcinolone acetonide in eyes with proliferative diabetic retinopathy. Ophthalmology. 2009;116:921–6.
Arjamaa O, Pöllönen M, Kinnunen K, Ryhänen T, Kaarniranta K. Increased IL-6 levels are not related to NF-κB or HIF-1α transcription factors activity in the vitreous of proliferative diabetic retinopathy. J Diabetes Complications. 2011;25:393–7.
Artini W, Gracia A, Kekalih A, Oktariana VD, Victor AA, Bani AP. Intravitreal anti-vascular endothelial growth factor injection combined with panretinal photocoagulation for neovascular glaucoma in Indonesian patients with diabetes mellitus: a prospective study. Med J Indones. 2019;28:258–67.
Asato R, Kita T, Kawahara S, Arita R, Mochizuki Y, Aiello LP, et al. Vitreous levels of soluble vascular endothelial growth factor receptor (VEGFR)-1 in eyes with vitreoretinal diseases. Br J Ophthalmol. 2011;95:1745–8.
Baharivand N, Zarghami N, Panahi F, Dokht Ghafari MY, Mahdavi Fard A, Mohajeri A. Relationship between vitreous and serum vascular endothelial growth factor levels, control of diabetes and microalbuminuria in proliferative diabetic retinopathy. Clin Ophthalmol. 2012;6:185–91.
Balogh A, Milibak T, Nagy ZZ, Resch MD. Molecular biological changes of the vitreous in different forms of retinal detachment. Invest Ophthalmol Vis Sci. 2019z;60:6607.
Balogh A, Milibák T, Szabó V, Nagy ZZ, Kaarniranta K, Resch MD. Immunological biomarkers of the vitreous responsible for proliferative alteration in the different forms of retinal detachment. BMC Ophthalmol. 2020;20:491.
Banerjee S, Savant V, Scott RA, Curnow SJ, Wallace GR, Murray PI. Multiplex bead analysis of vitreous humor of patients with vitreoretinal disorders. Invest Ophthalmol Vis Sci. 2007;48:2203–7.
Barile GR, Chang SS, Park LS, Reppucci VS, Schiff WM, Schmidt AM. Soluble cellular adhesion molecules in proliferative vitreoretinopathy and proliferative diabetic retinopathy. Curr Eye Res. 1999;19:219–27.
Barliya T, Dardik R, Nisgav Y, Dachbash M, Gaton D, Kenet G, et al. Possible involvement of NETosis in inflammatory processes in the eye: evidence from a small cohort of patients. Mol Vis. 2017;23:922–32.
Boehm BO, Lang G, Feldmann B, Kurkhaus A, Rosinger S, Volpert O, et al. Proliferative diabetic retinopathy is associated with a low level of the natural ocular anti-angiogenic agent pigment epithelium-derived factor (PEDF) in aqueous humor. a pilot study. Horm Metab Res. 2003;35:382–6.
Boulton M, Gregor Z, McLeod D, Charteris D, Jarvis-Evans J, Moriarty P, et al. Intravitreal growth factors in proliferative diabetic retinopathy: correlation with neovascular activity and glycaemic management. Br J Ophthalmol. 1997;81:228–33.
Bozkurt E, Çakır B, Çelik E, Doğan E, Uçak T, Alagöz G. Correlation of the aqueous humor total antioxidant capacity, total oxidant status, and levels of IL-6 and VEGF with diabetic retinopathy status. Arq Bras Oftalmol. 2019;82:136–40.
Bromberg-White JL, Glazer L, Downer R, Furge K, Boguslawski E, Duesbery NS. Identification of VEGF-independent cytokines in proliferative diabetic retinopathy vitreous. Invest Ophthalmol Vis Sci. 2013;54:6472–80.
Brzović-Šarić V, Landeka I, Šarić B, Barberić M, Andrijašević L, Cerovski B, et al. Levels of selected oxidative stress markers in the vitreous and serum of diabetic retinopathy patients. Mol Vis. 2015;21:649–64.
Burgos R, Simó R, Audí L, Mateo C, Mesa J, García-Ramírez M, et al. Vitreous levels of vascular endothelial growth factor are not influenced by its serum concentrations in diabetic retinopathy. Diabetologia. 1997;40:1107–9.
Byon IS, Jeon HS, Kim HW, Lee SJ, Lee JE, Oum BS. The effect of a systemic angiotensin receptor blocker on vascular endothelial growth factor in the vitreous of patients with proliferative diabetic retinopathy. Curr Eye Res. 2013;38:774–80.
Canataroglu H, Varinli I, Ozcan AA, Canataroglu A, Doran F, Varinli S. Interleukin (IL)-6, interleukin (IL)-8 levels and cellular composition of the vitreous humor in proliferative diabetic retinopathy, proliferative vitreoretinopathy, and traumatic proliferative vitreoretinopathy. Ocul Immunol Inflamm. 2005;13:375–81.
Cancarini A, Costagliola C, Dell’omo R, Romano M, Morescalchi F, Agnifili L, et al. Effect of intravitreal bevacizumab on serum, aqueous, and vitreous humor levels of erythropoietin in patients with proliferative diabetic retinopathy. Minerva Endocrinol. 2014;39:305–11.
Capeans C, De Rojas MV, Lojo S, Salorio MS. C-C chemokines in the vitreous of patients with proliferative vitreoretinopathy and proliferative diabetic retinopathy. Retina. 1998;18:546–50.
Chalam KV, Gupta S. Degree of capillary nonperfusion noted on wide-angle angiography directly correlate with intravitreal VEGF levels in proliferative vascular retinopathies. Invest Ophthalmol Vis Sci. 2019;60:6540.
Chen LY, Zhuo YH, Li YH, Huang XH, Zhang JL, Li SY, et al. Expression of stromal cell-derived factor-1 in diabetic retinopathy. Chin Med J (Engl). 2010;123:984–8.
Chen H, Zhang X, Liao N, Wen F. Increased levels of IL-6, sIL-6R, and sgp130 in the aqueous humor and serum of patients with diabetic retinopathy. Mol Vis. 2016;22:1005–14.
Chen H, Zhang X, Liao N, Wen F. Assessment of biomarkers using multiplex assays in aqueous humor of patients with diabetic retinopathy. BMC Ophthalmol. 2017;2:176.
Chen W, Lu Q, Lu L, Guan H. Increased levels of alphaB-crystallin in vitreous fluid of patients with proliferative diabetic retinopathy and correlation with vascular endothelial growth factor. Clin Exp Ophthalmol. 2017;45:379–84.
Chen H, Zhang X, Liao N, Mi L, Peng Y, Liu B, et al. Enhanced Expression of NLRP3 Inflammasome-Related Inflammation in Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2018;59:978–85.
Chen S, Zhong H, Wang Y, Wang Z, Liang X, Li S, et al. The clinical significance of long non-coding RNA ANRIL level in diabetic retinopathy. Acta Diabetol. 2020;57:409–18.
Chernykh VV, Varvarinsky EV, Smirnov EV, Chernykh DV, Trunov AN. Proliferative and inflammatory factors in the vitreous of patients with proliferative diabetic retinopathy. Indian J Ophthalmol. 2015;63:33–36.
Cho J, Jun G, Stein T, Chung K, McConnell, Fiorello M, et al. Vitreous cytokine levels in proliferative diabetic retinopathy and cataracts. Invest Ophthalmol Vis Sci. 2018;59:207.
Cicik E, Tekin H, Akar S, Ekmekçi OB, Donma O, Koldaş L, et al. Interleukin-8, nitric oxide and glutathione status in proliferative vitreoretinopathy and proliferative diabetic retinopathy. Ophthalmic Res. 2003;35:251–5.
Citirik M, Kabatas EU, Batman C, Akin KO, Kabatas N. Vitreous vascular endothelial growth factor concentrations in proliferative diabetic retinopathy versus proliferative vitreoretinopathy. Ophthalmic Res. 2012;47:7–12.
Comyn O, Bainbridge JWB. A pilot randomized controlled trial of ranibizumab pre-treatment for diabetic vitrectomy (The RaDiVit study). Invest Ophthalmol Vis Sci. 2014;55:2302.
Comyn O, Lange C, Bainbridge JWB. Vitreous and plasma cytokine levels in subjects with advanced proliferative diabetic retinopathy in the Ranibizumab in Diabetic Vitrectomy (RaDiVit) Study. Invest Ophthalmol Vis Sci. 2019;60:5362.
Cui L, Lu H. Alteration of intraocular pigment epithelium-derived factor and vascular endothelial growth factor in patients with diabetic retinopathy. Int Eye Sci. 2007;7:23–26.
Dai Y, Wu Z, Wang F, Zhang Z, Yu M. Identification of chemokines and growth factors in proliferative diabetic retinopathy vitreous. Biomed Res Int. 2014;2014:486386.
Dallinga M, Klaassen I, Van Geest RJ, Lesnik-Oberstein SY, Goldschmeding R, Van Noorden CJF, et al. A shift in the balance of Vegf and Ctgf by Bevacizumab Causes the Angio-Fibrotic Switch in Proliferative Diabetic Retinopathy. Eur J Ophthalmol. 2013;23:453.
Dan-Brezis I, Zahavi A, Axer-Siegel R, Nisgav Y, Dahbash M, Weinberger D, et al. Inflammation, angiogenesis and coagulation interplay in a variety of retinal diseases. Acta Ophthalmol. 2020;98:e559–e562.
De La Cadena RA, Rico MC, Del Carpio-Cano F, Safadi F, Kunapulo SP, Smith G, et al. Thrombospondin-1 (TSP1), transforming growth factor-beta (TGFb) and connective tissue growth factor (CTGF) are involved in the pathophysiology of diabetic retinopathy. Blood. 2011;118:3275.
Delil IS, Cekic O, Yaman A, Sirikci O, Haklar G. The discordance between serum and vitreous vascular endothelial growth factor levels in proliferative diabetic retinopathy. Clin Chem. 2016;62:s38.
Deng J, Wu DZ, Gao R. Elevated vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Yan Ke Xue Bao. 1999;15:17–21.
Duh EJ, Yang HS, Haller JA, De Juan E, Humayun MS, Gehlbach P, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor: implications for ocular angiogenesis. Am J Ophthalmol. 2004;137:668–74.
Ehrlich R, Zahavi A, Axer-Siegel R, Budnik I, Dreznik A, Dahbash M, et al. Correlation between interleukin-6 and thrombin-antithrombin III complex levels in retinal diseases. Curr Eye Res. 2017;42:1269–72.
Elner SG, Elner VM, Jaffe GJ, Stuart A, Kunkel SL, Strieter RM. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995;14:1045–53.
Elner SG, Strieter R, Bian ZM, Kunkel S, Mokhtarzaden L, Johnson M, et al. Interferon-induced protein 10 and interleukin 8. C-X-C chemokines present in proliferative diabetic retinopathy. Arch Ophthalmol. 1998;116:1597–601.
Endo M, Yanagisawa K, Tsuchida K, Okamoto T, Matsushita T, Higuchi M, et al. Increased levels of vascular endothelial growth factor and advanced glycation end products in aqueous humor of patients with diabetic retinopathy. Horm Metab Res. 2001;33:317–22.
Esser P, Bresgen M, Fischbach R, Heimann K, Wiedemann P. Intercellular adhesion molecule-1 levels in plasma and vitreous from patients with vitreoretinal disorders. Ger J Ophthalmol. 1995;4:269–74.
Feng S, Yu H, Yu Y, Geng Y, Li D, Yang C, et al. Levels of inflammatory cytokines IL-1β, IL-6, IL-8, IL-17A, and TNF-α in aqueous humour of patients with diabetic retinopathy. J Diabetes Res. 2018;2018:8546423.
Feng J, Li B, Wen J, Jiang Y. Preoperative timing of intravitreal bevacizumab injection for proliferative diabetic retinopathy patients. Ophthalmic Res. 2018;60:250–7.
Forooghian F, Kertes PJ, Eng KT, Agrón E, Chew EY. Alterations in the intraocular cytokine milieu after intravitreal bevacizumab. Invest Ophthalmol Vis Sci. 2010;51:2388–92.
Forooghian F, Kertes PJ, Eng KT, Albiani DA, Kirker AW, Merkur AB, et al. Alterations in intraocular cytokine levels following intravitreal ranibizumab. Can J Ophthalmol. 2016;51:87–90.
Fosmark DS, Bragadóttir R, Stene-Johansen I, Berg JP, Berg TJ, Lund T, et al. Increased vitreous levels of hydroimidazolone in type 2 diabetes patients are associated with retinopathy: a case-control study. Acta Ophthalmol Scand. 2007;85:618–22.
Freyberger H, Bröcker M, Yakut H, Hammer J, Effert R, Schifferdecker E, et al. Increased levels of platelet-derived growth factor in vitreous fluid of patients with proliferative diabetic retinopathy. Exp Clin Endocrinol Diabetes. 2000;108:106–9.
Fujita Y, Sato T, Someya H, Takayama K, Karasawa Y, Takeuchi M. The utility to evaluate inflammatory cytokines in the aqueous humor and aqueous flare in patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59:5373.
Funatsu H, Yamashita H, Nakanishi Y, Hori S. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy. Br J Ophthalmol. 2002;86:311–5.
Funatsu H, Yamashita H, Noma H, Mochizuki H, Mimura T, Ikeda T, et al. Outcome of vitreous surgery and the balance between vascular endothelial growth factor and endostatin. Invest Ophthalmol Vis Sci. 2003;44:1042–7.
Funatsu H, Yamashita H, Noma H, Mimura T, Sakata K, Hori S. Risk evaluation of outcome of vitreous surgery for proliferative diabetic retinopathy based on vitreous level of vascular endothelial growth factor and angiotensin II. Br J Ophthalmol. 2004;88:1064–8.
Funatsu H, Yamashita H, Mimura T, Noma H, Nakamura S, Hori S. Risk evaluation of outcome of vitreous surgery based on vitreous levels of cytokines. Eye (Lond). 2007;21:377–82.
Gao X, Ma K, Lu N, Xu Y, Hong T, Peng X. Elevated LRP6 levels correlate with vascular endothelial growth factor in the vitreous of proliferative diabetic retinopathy. Mol Vis. 2015;21:665–72.
Gao X, Ma K, Lu N, Hong T, Xu Y, Peng X. Correlation of increased intravitreous wnt3a with vascular endothelial growth factor in proliferative diabetic retinopathy. Retina. 2016;36:812–8.
Ghodasra DH, Fante R, Gardner TW, Langue M, Niziol LM, Besirli C, et al. Safety and feasibility of quantitative multiplexed cytokine analysis from office-based vitreous aspiration. Invest Ophthalmol Vis Sci. 2016;57:3017–23.
Gomaa AR, Elsayed ET, Moftah RF. MicroRNA-200b expression in the vitreous humor of patients with proliferative diabetic retinopathy. Ophthalmic Res. 2017;58:168–75.
Gucciardo E, Loukovaara S, Korhonen A, Repo P, Martins B, Vihinen H, et al. The microenvironment of proliferative diabetic retinopathy supports lymphatic neovascularization. J Pathol. 2018;245:172–85.
Gustavsson C, Agardh CD, Agardh E. Vitreous and aqueous concentrations of inflammatory mediators in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51:5610.
Gustavsson C, Agardh CD, Agardh E. Profile of intraocular tumour necrosis factor-α and interleukin-6 in diabetic subjects with different degrees of diabetic retinopathy. Acta Ophthalmol. 2013;91:445–52.
Hassan SJ, Babapoor S, Jee KJ, Puchner B, Deshpande M, Semenza GL, et al. Hypoxia inducible factor-1 upregulates the expression of plasminogen activator inhibitor-1 in retinal endothelial cells to promote their survival in proliferative diabetic retinopathy patients. Invest Ophthalmol Vis Sci. 2015;56:4282.
Hattenbach LO, Allers A, Gümbel HO, Scharrer I, Koch FH. Vitreous concentrations of TPA and plasminogen activator inhibitor are associated with VEGF in proliferative diabetic vitreoretinopathy. Retina. 1999;19:383–9.
Hattori T, Shimada H, Nakashizuka H, Mizutani Y, Mori R, Yuzawa M. Dose of intravitreal bevacizumab (Avastin) used as preoperative adjunct therapy for proliferative diabetic retinopathy. Retina. 2010;30:761–4.
Hernández C, Burgos R, Cantón A, García-Arumí J, Segura RM, Simó R. Vitreous levels of vascular cell adhesion molecule and vascular endothelial growth factor in patients with proliferative diabetic retinopathy: a case-control study. Diabetes Care. 2001;24:516–21.
Hernández C, Lecube A, Segura RM, Sararols L, Simó R. Nitric oxide and vascular endothelial growth factor concentrations are increased but not related in vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2002;19:655–60.
Hernández C, Segura RM, Fonollosa A, Carrasco E, Francisco G, Simó R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2005;22:719–22.
Hernández C, Ortega F, García-Ramírez M, Villarroel M, Casado J, García-Pascual L, et al. Lipopolysaccharide-binding protein and soluble CD14 in the vitreous fluid of patients with proliferative diabetic retinopathy. Retina. 2010;30:345–52.
Hines J, Ecker S, Glaser B. Sequential in-office vitreous aspirations demonstrate that up-regulation of matrix metalloproteinases may regulate angiogenesis in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54:1144.
Hogeboom van Buggenum IM, Polak BC, Reichert-Thoen JW, de Vries-Knoppert WA, van Hinsbergh VW, Tangelder GJ. Angiotensin converting enzyme inhibiting therapy is associated with lower vitreous vascular endothelial growth factor concentrations in patients with proliferative diabetic retinopathy. Diabetologia. 2002;45:203–9.
Hong F, Yang DY, Li L, Zheng YF, Wang XJ, Guo SRN, et al. Relationship between aqueous humor levels of cytokines and axial length in patients with diabetic retinopathy. Asia Pac J Ophthalmol (Philos). 2020;9:149–55.
Houssen ME, El-Hussiny MAB, El-Kannishy A, Sabry D, El Mahdy R, Shaker ME. Serum and aqueous humor concentrations of interleukin-27 in diabetic retinopathy patients. Int Ophthalmol. 2018;38:1817–23.
Hsu MY, Yang CY, Hsu WH, Lin KH, Wang CY, Shen YC, et al. Monitoring the VEGF level in aqueous humor of patients with ophthalmologically relevant diseases via ultrahigh sensitive paper-based ELISA. Biomaterials. 2014;35:3729–35.
Hua A, Johnson W, Nandakumar N, Teague GC, Baldwin ME, Lashkari K. Linear discriminate analysis for design of high-value biomarkers in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58:2511.
Huber M, Wachtlin J. Vitreous levels of proteins implicated in angiogenesis are modulated in patients with retinal or choroidal neovascularization. Ophthalmologica. 2012;228:188–93.
Igbre A, Ecker S, Hines J, Glaser B. In-office vitreous sampling shows that levels of MMP-9 correlate with the severity of proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54:276.
Inafuku S, Noda K, Amano M, Nishimura S, Ishida S. Short communication: increase of sialylated N-glycansin eyes with neovascular glaucoma secondary to proliferative diabetic retinopathy. Curr Eye Res. 2016;41:721–4.
Inomata Y, Hirata A, Takahashi E, Kawaji T, Fukushima M, Tanihara H. Elevated erythropoietin in vitreous with ischemic retinal diseases. Neuroreport. 2004;15:877–9.
Ishikawa K, Kohno RI, Mori K, Murakami Y, Nakao S, Akiyama M, et al. Increased expression of periostin and tenascin-C in eyes with neovascular glaucoma secondary to PDR. Graefes Arch Clin Exp Ophthalmol. 2020;258:621–8.
Ishizaki E, Takai S, Ueki M, Maeno T, Maruichi M, et al. Correlation between angiotensin-converting enzyme, vascular endothelial growth factor, and matrix metalloproteinase-9 in the vitreous of eyes with diabetic retinopathy. Am J Ophthalmol. 2006;141:129–34.
Itakura H, Kishi S, Kotajima N, Murakami M. Persistent secretion of vascular endothelial growth factor into the vitreous cavity in proliferative diabetic retinopathy after vitrectomy. Ophthalmology. 2004;111:1880–4.
Izuta H, Chikaraishi Y, Adachi T, Shimazawa M, Sugiyama T, Ikeda T, et al. Extracellular SOD and VEGF are increased in vitreous bodies from proliferative diabetic retinopathy patients. Mol Vis. 2009;15:2663–72.
Izuta H, Matsunaga N, Shimazawa M, Sugiyama T, Ikeda T, Hara H. Proliferative diabetic retinopathy and relations among antioxidant activity, oxidative stress, and VEGF in the vitreous body. Mol Vis. 2010;16:130–6.
Jeon S, Lee WK. Intravitreal bevacizumab increases intraocular interleukin-6 levels at 1 day after injection in patients with proliferative diabetic retinopathy. Cytokine. 2012;60:535–9.
Jiang T, Gu J, Zhang P, Chen W, Chang Q. The effect of adjunctive intravitreal conbercept at the end of diabetic vitrectomy for the prevention of post-vitrectomy hemorrhage in patients with severe proliferative diabetic retinopathy: a prospective, randomized pilot study. BMC Ophthalmol. 2020;20:43.
Jiang F, Chen Q, Huang L, Wang Y, Zhang Z, Meng X. TNFSF15 Inhibits Blood Retinal Barrier Breakdown Induced by Diabetes. Int J Mol Sci. 2016;17:615.
Jung BJ, Lee MY, Jeon S. Systemic Factors Related to Intraocular Levels of Interleukin-6 and Vascular Endothelial Growth Factor in Diabetic Retinopathy. J Ophthalmol. 2019;2019:4831967.
Kahtani EA, Xu Z, Al Rashaed S, Wu L, Mahale A, Tian J. Vitreous levels of placental growth factor correlate with activity of proliferative diabetic retinopathy and are not influenced by bevacizumab treatment. Eye (Lond). 2017;31:529–36.
Kakehashi A, Inoda S, Mameuda C, Kuroki M, Jono T, Nagai R, et al. Relationship among VEGF, VEGF receptor, AGEs, and macrophages in proliferative diabetic retinopathy. Diabetes Res Clin Pr. 2008;79:438–45.
Kanda A, Noda K, Saito W, Ishida S. (Pro)renin receptor is associated with angiogenicactivity in proliferative diabetic retinopathy. Diabetologia. 2012;55:3104–13.
Katagiri M, Shoji J, Kato S, Kitano S, Uchigata Y. Relationships between vitreous levels of soluble receptor for advanced glycation end products (sRAGE) and renal function in patients with diabetic retinopathy. Int Ophthalmol. 2017;37:1247–55.
Katagiri M, Shoji J, Inada N, Kato S, Kitano S, Uchigata Y. Evaluation of vitreous levels of advanced glycation end products and angiogenic factors as biomarkers for severity of diabetic retinopathy. Int Ophthalmol. 2018;38:607–15.
Katome T, Namekata K, Naito T, Semba K, Guo X, Harada C, et al. Expression of promyelocytic leukemia protein and vascular endothelial growth factor in aqueous humor and vitreous fluid in patients with proliferative diabetic retinopathy. Diabetes Res Clin Pr. 2012;98:e9–e11.
Katome T, Namekata K, Mitamura Y, Semba K, Egawa M, Naito T, et al. Expression of intraocular peroxisome proliferator-activated receptor gamma in patients with proliferative diabetic retinopathy. J Diabetes Complications. 2015;29:275–81.
Katsura Y, Okano T, Noritake M, Kosano H, Nishigori H, Kado S, et al. Hepatocyte growth factor in vitreous fluid of patients with proliferative diabetic retinopathy and other retinal disorders. Diabetes Care. 1998;21:1759–63.
Katsura Y, Okano T, Matsuno K, Osako M, Kure M, Watanabe T, et al. Erythropoietin is highly elevated in vitreous fluid of patients with proliferative diabetic retinopathy. Diabetes Care. 2005;28:2252–4.
Kauffmann DJ, van Meurs JC, Mertens DA, Peperkamp E, Master C, Gerritsen ME. Cytokines in vitreous humor: interleukin-6 is elevated in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1994;35:900–6.
Kaviarasan K, Jithu M, Arif Mulla M, Sharma T, Sivasankar S, Das UN, et al. Low blood and vitreal BDNF, LXA4 and altered Th1/Th2 cytokine balance are potential risk factors for diabetic retinopathy. Metabolism. 2015;64:958–66.
Kawashima M, Shoji J, Nakajima M, Kamura Y, Sato Y. Soluble IL-6 receptor in vitreous fluid of patients with proliferative diabetic retinopathy. Jpn J Ophthalmol. 2007;51:100–4.
Keles A, Sonmez K, Erol YO, Ayyıldız SN, Ogus E. Vitreous levels of vascular endothelial growth factor, stromal cell-derived factor-1α, and angiopoietin-like protein 2 in patients with active proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2021;259:53–60.
Khaliq A, Foreman D, Ahmed A, Weich H, Gregor Z, McLeod D, et al. Increased expression of placenta growth factor in proliferative diabetic retinopathy. Lab Invest. 1998;78:109–16.
Kinnunen K, Puustjärvi T, Teräsvirta M, Nurmenniemi P, Heikura T, Laidinen S, et al. Differences in retinal neovascular tissue and vitreous humour in patients with type 1 and type 2 diabetes. Br J Ophthalmol. 2009;93:1109–15.
Kita T, Clermont AC, Murugesan N, Zhou Q, Fujisawa K, Ishibashi T, et al. Plasma Kallikrein-Kinin system as a VEGF-independent mediator of diabetic macular edema. Diabetes. 2015;64:3588–99.
Klaassen I, de Vries EW, Vogels IMC, van Kampen AHC, Bosscha MI, Steel DHW, et al. Identification of proteins associated with clinical and pathological features of proliferative diabetic retinopathy in vitreous and fibrovascular membranes. PLoS One. 2017;12:e0187304.
Klaassen I, Steel DHW, Schlingemann RO. Differential protein levels in vitreous of proliferative diabetic retinopathy patients treated with aflibercept and bevacizumab. Eur J Ophthalmol. 2019;29:NP15.
Kobayashi T, Machida S, Fujiwara T, Ishibe T, Kurosaka D. Vitreous levels of vascular endothelial growth factor in eyes with anterior hyaloidal fibrovascular proliferation. Clin Ophthalmol. 2010;4:1043–6.
Kobayashi Y, Yoshida S, Nakama T, Zhou Y, Ishikawa K, Arita R, et al. Overexpression of CD163 in vitreous and fibrovascular membranes of patients with proliferative diabetic retinopathy: possible involvement of periostin. Br J Ophthalmol. 2015;99:451–6.
Kobayashi Y, Yoshida S, Zhou Y, Nakama T, Ishikawa K, Arima M, et al. Tenascin-C promotes angiogenesis in fibrovascular membranes in eyes with proliferative diabetic retinopathy. Mol Vis. 2016;22:436–45.
Kojima S, Yamada T, Tamai M. Quantitative analysis of interleukin-6 in vitreous from patients with proliferative vitreoretinal diseases. Jpn J Ophthalmol. 2001;45:40–45.
Kokubun T, Tsuda S, Kunikata H, Yasuda M, Himori N, Kunimatsu-Sanuki S, et al. Characteristic profiles of inflammatory cytokines in the aqueous humor of glaucomatous eyes. Ocul Immunol Inflamm. 2018;26:1177–88.
Kosano H, Okano T, Katsura Y, Noritake M, Kado S, Matsuoka T, et al. ProMMP-9 (92 kDa gelatinase) in vitreous fluid of patients with proliferative diabetic retinopathy. Life Sci. 1999;64:2307–15.
Koskela UE, Kuusisto SM, Nissinen AE, Savolainen MJ, Liinamaa MJ. High vitreous concentration of IL-6 and IL-8, but not of adhesion molecules in relation to plasma concentrations in proliferative diabetic retinopathy. Ophthalmic Res. 2013;49:108–14.
Kovacs K, Marra KV, Yu G, Wagley S, Ma J, Teague GC, et al. Angiogenic and inflammatory vitreous biomarkers associated with increasing levels of retinal ischemia. Invest Ophthalmol Vis Sci. 2015;56:6523–30.
Kubota R, Jhaveri C, Koester JM, Gregory JK. Effects of emixustat hydrochloride in patients with proliferative diabetic retinopathy: a randomized, placebo-controlled phase 2 study. Graefes Arch Clin Exp Ophthalmol. 2021;259:369–78.
Kuiper EJ, Van Nieuwenhoven FA, de Smet MD, van Meurs JC, Tanck MW, Oliver N. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One. 2008;3:e2675.
Kuzmin A, Lipatov D, Chistyakov T, Smirnova O, Arbuzova M, Ilin A, et al. Vascular endothelial growth factor in anterior chamber liquid patients with diabetic retinopathy, cataract and neovascular glaucoma. Ophthalmol Ther. 2013;2:41–51.
Lange CAK, Stavrakas P, Luhmann UFO, de Silva DJ, Ali RR, Gregor ZJ, et al. Intraocular oxygen distribution in advanced proliferative diabetic retinopathy. Am J Ophthalmol. 2011;152:406–12e3.
Li JK, Wei F, Jin XH, Dai YM, Cui HS, Li YM. Changes in vitreous VEGF, bFGF and fibrosis in proliferative diabetic retinopathy after intravitreal bevacizumab. Int J Ophthalmol. 2015;8:1202–6.
Li J, Hu WC, Song H, Lin JN, Tang X. Increased Vitreous Chemerin Levels Are Associated with Proliferative Diabetic Retinopathy. Ophthalmologica. 2016;236:61–66.
Li Y, Zhou T, Hsu A, Edwards PA, Gao H, Qiao X. Metformin reduces inflammation in diabetic human vitreous by activating AMPK and inhibiting NFκB signaling pathway. Invest Ophthalmol Vis Sci. 2019;60:6548.
Li B, Li MD, Ye JJ, Chen Z, Guo ZJ, Di Y. Vascular endothelial growth factor concentration in vitreous humor of patients with severe proliferative diabetic retinopathy after intravitreal injection of conbercept as an adjunctive therapy for vitrectomy. Chin Med J. 2020;133:664–9.
Liinamaa MJ, Savolainen MJ. High vitreous concentration of vascular endothelial growth factor in diabetic patients with proliferative retinopathy using statins. Ann Med. 2008;40:209–14.
Limb GA, Hickman-Casey J, Hollifield RD, Chignell AH. Vascular adhesion molecules in vitreous from eyes with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 1999;40:2453–7.
Limb GA, Hollifield RD, Webster L, Charteris DG, Chignell AH. Soluble TNF receptors in vitreoretinal proliferative disease. Invest Ophthalmol Vis Sci. 2001;42:1586–91.
Liu J, Xia X, Xiong S, Le Y, Xu H. Intravitreous high expression level of netrin-1 in patients with proliferative diabetic retinopathy. Eye Sci. 2011;26:35–42.
Liu R, Liu CM, Cui LL, Zhou L, Li N, Wei XD. Expression and significance of MiR-126 and VEGF in proliferative diabetic retinopathy. Eur Rev Med Pharm Sci. 2019;23:6387–93.
Liu Q, Zie P, Yuan S, Hu Z. Day Regimes of CONbercept on CytokinEs of PDR Patients Undergoing Vitrectomy Trial (CONCEPT): Effects of preoperative intravitreal conbercept on cytokines in the vitreous with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2019;60:5372.
Loukovaara S, Robciuc A, Holopainen JM, Lehti K, Pessi T, Liinamaa J, et al. Ang-2 upregulation correlates with increased levels of MMP-9, VEGF, EPO and TGFβ1 in diabetic eyes undergoing vitrectomy. Acta Ophthalmol. 2013;91:531–9.
Loukovaara S, Koivunen P, Inglés M, Escobar J, Vento M, Andersson S. Elevated protein carbonyl and HIF-1α levels in eyes with proliferative diabetic retinopathy. Acta Ophthalmol. 2014;92:323–7.
Loukovaara S, Piippo N, Kinnunen K, Hytti M, Kaarniranta K, Kauppinen A. NLRP3 inflammasome activation is associated with proliferative diabetic retinopathy. Acta Ophthalmol. 2017;95:803–8.
Lu Q, Zou W, Chen B, Zou C, Zhao M, Zheng Z. ANGPTL-4 correlates with vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254:1281–8.
Lu Q, Lu L, Chen W, Lu P. Expression of angiopoietin-like protein 8 correlates with VEGF in patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255:1515–23.
Ma Y, Tao Y, Lu Q, Jiang YR. Intraocular expression of serum amyloid a and interleukin-6 in proliferative diabetic retinopathy. Am J Ophthalmol. 2011;152:678–85. e2
Ma Y, Zhang Y, Zhao T, Jiang YR. Vascular endothelial growth factor in plasma and vitreous fluid of patients with proliferative diabetic retinopathy patients after intravitreal injection of bevacizumab. Am J Ophthalmol. 2012;153:307–13. e2
Malik RA, Li C, Aziz W, Olson JA, Vohra A, McHardy KC, et al. Elevated plasma CD105 and vitreous VEGF levels in diabetic retinopathy. J Cell Mol Med. 2005;9:692–7.
Mallmann F, Canani LH. Intravitreal neurodegenerative and inflammatory mediators in proliferative diabetic retinopathy. Arq Bras Oftalmol. 2019;82:275–82.
Mandava N, Tirado-Gonzalez V, Geiger MD, Patnaik JL, Frazer-Abel A, Lynch AM, et al. Complement activation in the vitreous of patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2020;61:39.
Mao C, Yan H. Roles of elevated intravitreal IL-1β and IL-10 levels in proliferative diabetic retinopathy. Indian J Ophthalmol. 2014;62:699–701.
Marek N, Raczyńska K, Siebert J, Myśliwiec M, Zorena K, Myśliwska J, et al. Decreased angiogenin concentration in vitreous and serum in proliferative diabetic retinopathy. Microvasc Res. 2011;82:1–5.
Marra KV, Kovacs K, Wagley S, Akella S, Teague GC, Johnson W, et al. Placental growth factor levels correlated with retinal ischemia progression. Invest Ophthalmol Vis Sci. 2014;55:1909.
Masuda T, Shimazawa M, Hashimoto Y, Kojima A, Nakamura S, Suemori S, et al. Apolipoprotein E2 and E3, but Not E4, promote retinal pathologic neovascularization. Invest Ophthalmol Vis Sci. 2017;58:1208–17.
Matsumoto M, Suzuma K, Maki T, Kinoshita H, Tsuiki E, Fujikawa A, et al. Succinate increases in the vitreous fluid of patients with active proliferative diabetic retinopathy. Am J Ophthalmol. 2012;53:896–902. e1
Matsunaga N, Chikaraishi Y, Izuta H, Ogata N, Shimazawa M, Matsumura M, et al. Role of soluble vascular endothelial growth factor receptor-1 in the vitreous in proliferative diabetic retinopathy. Ophthalmology. 2008;115:1916–22.
Matsuyama K, Ogata N, Jo N, Shima C, Matsuoka M, Matsumura M. Levels of vascular endothelial growth factor and pigment epithelium-derived factor in eyes before and after intravitreal injection of bevacizumab. Jpn J Ophthalmol. 2009;53:243–8.
Matsuyama K, Ogata N, Matsuoka M, Wada M, Nishimura T, Takahashi K. Effects of intravitreally injected bevacizumab on vascular endothelial growth factor in fellow eyes. J Ocul Pharm Ther. 2011;27:379–83.
Megarity H, Deshpande M, Babapoor-Farrokhran S, Puchner B, Seemenza G, Montaner S, et al. Hypoxia-inducible factor 1-regulated angiogenic cytokines in prolifeartive diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58:4050.
Merlak M, Kovacević D, Balog T, Marotti T, Misljenović T, Mikulicić M, et al. Expression of vascular endothelial growth factor in proliferative diabetic retinopathy. Coll Antropol. 2008;32:39–43.
Mesquita J, Castro-de-Sousa JP, Rocha AS, Monteiro J, Passarinha L, Tomaz C. Quantification and comparison of VEGF-B in the vitreous of patients with diabetic ocular disease and a control group of patients with non-diabetic ocular disease. Invest Ophthalmol Vis Sci. 2014;55:399.
Mesquita J, Castro-de-Sousa JP, Tavares-Ratado P, Vaz-Pereira S, Neves A, Rocha AS, et al. Comparison of serum and vitreous PlGF in diabetic retinopathy patients and non-diabetic patients. Invest Ophthalmol Vis Sci. 2015;56:5179.
Mesquita J, Castro-de-Sousa JP, Vaz-Pereira S, Neves A, Passarinha L, Tomaz C. Quantitative analysis and correlation of VEGF-A and VEGF-B in serum and vitreous humor of patients with proliferative vs non-proliferative ocular disease. Invest Ophthalmol Vis Sci. 2017;58:604.
Mesquita J, Castro-de-Sousa JP, Vaz-Pereira S, Neves A, Passarinha LA, Tomaz CT. Evaluation of the growth factors VEGF-a and VEGF-B in the vitreous and serum of patients with macular and retinal vascular diseases. Growth Factors. 2018;36:48–57.
Mitamura Y, Takeuchi S, Matsuda A, Tagawa Y, Mizue Y, Nishihira J. Macrophage migration inhibitory factor levels in the vitreous of patients with proliferative diabetic retinopathy. Br J Ophthalmol. 2000;84:636–9.
Mitamura Y, Takeuchi S, Matsuda A, Tagawa Y, Mizue Y, Nishihira J. Monocyte chemotactic protein-1 in the vitreous of patients with proliferative diabetic retinopathy. Ophthalmologica. 2001;215:415–8.
Mocan MC, Kadayifcilar S, Eldem B. Elevated intravitreal interleukin-6 levels in patients with proliferative diabetic retinopathy. Can J Ophthalmol. 2006;41:747–52.
Mohammad G, Alam K, Nawaz MI, Siddiquei MM, Mousa A, Abu El-Asrar AM. Mutual enhancement between high-mobility group box-1 and NADPH oxidase-derived reactive oxygen species mediates diabetes-induced upregulation of retinal apoptotic markers. J Physiol Biochem. 2015;71:359–72.
Mohan N, Monickaraj F, Balasubramanyam M, Rema M, Mohan V. Imbalanced levels of angiogenic and angiostatic factors in vitreous, plasma and postmortem retinal tissue of patients with proliferative diabetic retinopathy. J Diabetes Complications. 2012;26:435–41.
Muhiddin HS, Kamaruddin MI, Ichsan AM, Budu. Vitreous and serum concentrations of vascular endothelial growth factor and platelet-derived growth factor in proliferative diabetic retinopathy. Clin Ophthalmol. 2020;14:1547–52.
Muramatsu D, Wakabayashi Y, Usui Y, Okunuki Y, Kezuka T, Goto H. Correlation of complement fragment C5a with inflammatory cytokines in the vitreous of patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2013;251:15–17.
Murata M, Noda K, Fukuhara J, Kanda A, Kase S, Saito W, et al. Soluble vascular adhesion protein-1 accumulates in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2012;53:4055–62.
Murata M, Noda K, Yoshida S, Saito M, Fujiya A, Kanda A, Ishida S. Unsaturated aldehyde acrolein promotes retinal glial cell migration. Invest Ophthalmol Vis Sci. 2019;60:4425–35.
Murugeswari P, Shukla D, Rajendran A, Kim R, Namperumalsamy P, Muthukkaruppan V. Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and eales’ disease. Retina. 2008;28:817–24.
Murugeswari P, Shukla D, Kim R, Namperumalsamy P, Stitt AW, Muthukkaruppan V. Angiogenic potential of vitreous from proliferative diabetic retinopathy and Eales’ disease patients. PLoS One. 2014;9:e107551.
Mutlu M, Yuksel N, Takmaz T, Dincel AS, Bilgihan A, Altınkaynak H. Aqueous humor pentraxin-3 levels in patients with diabetes mellitus. Eye. 2017;31:1463–7.
Nakamura N, Hasegawa G, Obayashi H, Yamazaki M, Ogata M, Nakano K. Increased concentration of pentosidine, an advanced glycation end product, and interleukin-6 in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Res Clin Pr. 2003;61:93–101.
Nakamura S, Iwasaki N, Funatsu H, Kitano S, Iwamoto Y. Impact of variants in the VEGF gene on progression of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2009;247:21–26.
Nakamura S, Morimoto N, Tsuruma K, Izuta H, Yasuda Y, Kato N, et al. Tissue kallikrein inhibits retinal neovascularization via the cleavage of vascular endothelial growth factor-165. Arterioscler Thromb Vasc Biol. 2011;31:1041–8.
Nakamura S, Shimazawa M, Inoue Y, Takata S, Ito Y, Tsuruma K, et al. Role of metallothioneins 1 and 2 in ocular neovascularization. Invest Ophthalmol Vis Sci. 2014;55:6851–60.
Nam DH, Oh J, Roh JH, Huh K. Different expression of vascular endothelial growth factor and pigment epithelium-derived factor between diabetic and non-diabetic epiretinal membranes. Ophthalmologica. 2009;223:188–91.
Nandakumar N, Teague GC, Lopez FJ, Johnson W, Lashkari K. Evaluation of vitreous factor in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57:5452.
Nawaz MI, Van Raemdonck K, Mohammad G, Kangave D, Van Damme J, Abu, El-Asrar AM, et al. Autocrine CCL2, CXCL4, CXCL9 and CXCL10 signal in retinal endothelial cells and are enhanced in diabetic retinopathy. Exp Eye Res. 2013;109:67–76.
Nezu N, Usui Y, Asakage M, Shimizu H, Ogawa M, Yamakawa N, et al. Determining immune-related factors of intraocular diseases by artificial intelligence methods. Invest Ophthalmol Vis Sci. 2019;60:5369.
Nicoletti R, Venza I, Ceci G, Visalli M, Teti D, Reibaldi A. Vitreous polyamines spermidine, putrescine, and spermine in human proliferative disorders of the retina. Br J Ophthalmol. 2003;87:1038–42.
Nishiguchi KM, Ushida H, Tomida D, Kachi S, Kondo M, Terasaki H. Age-dependent alteration of intraocular soluble heparan sulfate levels and its implications for proliferative diabetic retinopathy. Mol Vis. 2013;19:1125–31.
Nisic F, Jovanovic N, Mavija M, Alimanovic-Halilovic E, Nisic A, Lepara O, et al. Vitreous concentrations of vascular endothelial growth factor as a potential biomarker for postoperative complications following pars plana vitrectomy. Arch Med Sci. 2019;15:449–56.
Ogata N, Nishikawa M, Nishimura T, Mitsuma Y, Matsumura M. Unbalanced vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in diabetic retinopathy. Am J Ophthalmol. 2002;134:348–53.
Oh IK, Kim SW, Oh J, Lee TS, Huh K. Inflammatory and angiogenic factors in the aqueous humor and the relationship to diabetic retinopathy. Curr Eye Res. 2010;35:1116–27.
Oubaha M, Miloudi K, Dejda A, Guber V, Mawambo G, Germain MA, et al. Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathy. Sci Transl Med. 2016;8:362ra144.
Ozaki H, Katsura Y, Noritake M, Matsuno K, Okano T, Kosano H, et al. Effects of endostatin in proliferative diabetic retinopathy. J Tokyo Med Univ. 2003;61:226–31.
Parveen N, Qureshi IZ. Vascular endothelial growth factor, interleukin 6 and leptin in the serum and vitreous fluid of type 2 diabetic patients. J Med Sci. 2013;21:40–43.
Patel JI, Hykin PG, Gregor ZJ, Boulton M, Cree IA. Angiopoietin concentrations in diabetic retinopathy. Br J Ophthalmol. 2005;89:480–3.
Patel JI, Hykin PG, Cree IA. Diabetic cataract removal: postoperative progression of maculopathy-growth factor and clinical analysis. Br J Ophthalmol. 2006;90:697–701.
Patel JI, Tombran-Tink J, Hykin PG, Gregor ZJ, Cree IA. Vitreous and aqueous concentrations of proangiogenic, antiangiogenic factors and other cytokines in diabetic retinopathy patients with macular edema: Implications for structural differences in macular profiles. Exp Eye Res. 2006;82:798–806.
Patel JI, Saleh GM, Hykin PG, Gregor ZJ, Cree IA. Concentration of haemodynamic and inflammatory related cytokines in diabetic retinopathy. Eye. 2008;22:223–8.
Peng M, Hemarat K, Liu Q, Kong X, Sukpen I, Kudish M. Feasibility of detecting RNA expression in human vitreous. Invest Ophthalmol Vis Sci. 2018;59:334.
Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia. 2005;48:2422–7.
Petrovic MG, Korosec P, Kosnik M, Hawlina M. Vitreous levels of interleukin-8 in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2007;143:175–6.
Petrovič MG, Korošec P, Košnik M, Hawlina M. Association of preoperative vitreous IL-8 and VEGF levels with visual acuity after vitrectomy in proliferative diabetic retinopathy. Acta Ophthalmol. 2010;88:e311–316.
Ponnalagu M, Kim R, Shukla D, Namperumalsamy P, Muthukkaruppan VR, Stitt AW. Angiogenesis induced by proliferative diabetic retinopathy and eales’ disease vitreous is mediated by a common pro-inflammatory mechanism. Invest Ophthalmol Vis Sci. 2010;51:68.
Praidou A, Klangas I, Papakonstantinou E, Androudi S, Georgiadis N, Karakiulakis G, et al. Vitreous and serum levels of platelet-derived growth factor and their correlation in patients with proliferative diabetic retinopathy. Curr Eye Res. 2009;34:152–61.
Praidou A, Papakonstantinou E, Androudi S, Georgiadis N, Karakiulakis G, Dimitrakos S. Vitreous and serum levels of vascular endothelial growth factor and platelet-derived growth factor and their correlation in patients with non-proliferative diabetic retinopathy and clinically significant macula oedema. Acta Ophthalmol. 2011;89:248–54.
Qian J, Lu Q, Tao Y, Jiang YR. Vitreous and plasma concentrations of apelin and vascular endothelial growth factor after intravitreal bevacizumab in eyes with proliferative diabetic retinopathy. Retina. 2011;31:161–8.
Ra H, Park JH, Baek JU, Baek J. Relationships among retinal nonperfusion, neovascularization, and vascular endothelial growth factor levels in quiescent proliferative diabetic retinopathy. J Clin Med. 2020;9:1462.
Raczyńska D, Lisowska KA, Pietruczuk K, Borucka J, Ślizień M, Raczyńska K, et al. The level of cytokines in the vitreous body of severe proliferative diabetic retinopathy patients undergoing posterior vitrectomy. Curr Pharm Des. 2018;24:3276–81.
Raiser R, Artunary O, Gormus U, Yuzbasioglu E, Sengul A, Kukner AS, et al. Interleukin-8 and tumor necrosis factor-alpha levels in vitreous samples from patients with diabetic retinopathy. Int Eye Sci. 2013;13:2365–9.
Ran R, Du L, Zhang X, Chen X, Fang Y, Li Y, et al. Elevated hydrogen sulfide levels in vitreous body and plasma in patients with proliferative diabetic retinopathy. Retina. 2014;34:2003–9.
Reverter JL, Nadal J, Fernández-Novell JM, Ballester J, Ramió-Lluch L, Rivera MM, et al. Tyrosine phosphorylation of vitreous inflammatory and angiogenic peptides and proteins in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:1378–82.
Reverter JL, Nadal J, Ballester J, Ramió-Lluch L, Rivera MM, Fernández-Novell JM, et al. Diabetic retinopathy is associated with decreased tyrosine nitrosylation of vitreous interleukins IL-1α, IL-1β, and IL-7. Ophthalmic Res. 2011;46:169–74.
Rezzola S, Nawaz MI, Cancarini A, Semeraro F, Presta M. Vascular endothelial growth factor in the vitreous of proliferative diabetic retinopathy patients: chasing a hiding prey? Diabetes Care. 2019;42:e105–e106.
Ridano ME, Subirada PV, Paz MC, Lorenc VE, Stupirski JC, Gramajo AL, et al. Galectin-1 expression imprints a neurovascular phenotype in proliferative retinopathies and delineates responses to anti-VEGF. Oncotarget. 2017;8:32505–22.
Rollin R, Mediero A, Martínez-Montero JC, Roldán-Pallarés M, Suárez-Leoz M, Vidal-Fernández P, et al. Atrial natriuretic peptide in the vitreous humor and epiretinal membranes of patients with proliferative diabetic retinopathy. Mol Vis. 2004;10:450–7.
Rusnak S, Vrzalova J, Sobotova M, Hecova L, Ricarova R, Topolcan O. The measurement of intraocular biomarkers in various stages of proliferative diabetic retinopathy using multiplex xMAP technology. J Ophthalmol. 2015;2015:424783.
Sahajpal NS, Jain S, Vig V, Singh P, Singh R, Singh H, et al. Insulin in the vitreous humor of patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59:5366.
Sahajpal NS, Jain S, Vig V, Singh P, Singh R, Singh H, et al. Increased vitreous VEGF: Correlation with insulin and other plasma metabolites in patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2019;60:2675.
Sakamoto T, Ito S, Yoshikawa H, Hata Y, Ishibashi T, Sueishi K, et al. Tissue factor increases in the aqueous humor of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2001;239:865–71.
Sassa Y, Yoshida S, Ishikawa K, Asato R, Ishibashi T, Kono T. The kinetics of VEGF and MCP-1 in the second vitrectomy cases with proliferative diabetic retinopathy. Eye. 2016;30:746–53.
Sawada O, Kawamura H, Kakinoki M, Ohji M. Vascular endothelial growth factor in fellow eyes of eyes injected with intravitreal bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2008;246:1379–81.
Schoenberger SD, Kim SJ, Sheng J, Rezaei KA, Lalezary M, Cherney E. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Invest Ophthalmol Vis Sci. 2012;53:5906–11.
Schoenberger SD, Kim SJ, Shah R, Sheng J, Cherney E. Reduction of interleukin 8 and platelet-derived growth factor levels by topical ketorolac, 0.45%, in patients with diabetic retinopathy. JAMA Ophthalmol. 2014;132:32–37.
Schwartzman ML, Iserovich P, Gotlinger K, Bellner L, Dunn MW, Sartore M, et al. Profile of lipid and protein autacoids in diabetic vitreous correlates with the progression of diabetic retinopathy. Diabetes. 2010;59:1780–8.
Schori C, Trachsel C, Grossmann J, Zygoula I, Barthelmes D, Grimm C. The proteomic landscape in the vitreous of patients with age-related and diabetic retinal disease. Invest Ophthalmol Vis Sci. 2018;59:AMD31–AMD40.
Selim KM, Sahan D, Muhittin T, Osman C, Mustafa O. Increased levels of vascular endothelial growth factor in the aqueous humor of patients with diabetic retinopathy. Indian J Ophthalmol. 2010;58:375–9.
Semeraro F, Cancarini A, Morescalchi F, Romano MR, dell’Omo R, Ruggeri G, et al. Serum and intraocular concentrations of erythropoietin and vascular endothelial growth factor in patients with type 2 diabetes and proliferative retinopathy. Diabetes Metab. 2014;40:445–51.
Shahulhameed S, Vishwakarma S, Chhablani J, Tyagi M, Pappuru RR, Jakati S, et al. A systematic investigation on complement pathway activation in diabetic retinopathy. Front Immunol. 2020;11:154.
Shchuko AG, Zaytseva NV, & Zlobin IV. Intraocular cytokines concentrations in patients with different levels of activity of retinal pathologies. Ophthalmologica. 2014;232:29
Shen Y, Cao H, Chen F, Suo Y, Wang N, Xu X. A cross-sectional study of vitreous and serum high mobility group box-1 levels in proliferative diabetic retinopathy. Acta Ophthalmol. 2020;98:e212–e216.
Shimizu H, Kaneko H, Suzumura A, Takayama K, Namba R, Funahashi Y, et al. Biological characteristics of subsilicone oil fluid and differences with other ocular humors. Transl Vis Sci Technol. 2019;8:28.
Shimura M, Yasuda K, Nakazawa T, Abe T, Shiono T, Iida T, et al. Panretinal photocoagulation induces pro-inflammatory cytokines and macular thickening in high-risk proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2009;247:1617–24.
Shinoda K, Ishida S, Kawashima S, Wakabayashi T, Matsuzaki T, Takayama M, et al. Comparison of the levels of hepatocyte growth factor and vascular endothelial growth factor in aqueous fluid and serum with grades of retinopathy in patients with diabetes mellitus. Br J Ophthalmol. 1999;83:834–7.
Shinoda K, Ishida S, Kawashima S, Wakabayashi T, Uchita M, Matsuzaki T, et al. Clinical factors related to the aqueous levels of vascular endothelial growth factor and hepatocyte growth factor in proliferative diabetic retinopathy. Curr Eye Res. 2000;21:655–61.
Simó R, Lecube A, Segura RM, García Arumí J, Hernández C. Free insulin growth factor-I and vascular endothelial growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2002;134:376–82.
Simó R, Vidal MT, García-Arumí J, Carrasco E, García-Ramírez M, Segura RM, et al. Intravitreous hepatocyte growth factor in patients with proliferative diabetic retinopathy: a case-control study. Diabetes Res Clin Pr. 2006;71:36–44.
Sohn EH, He S, Kim LA, Salehi-Had H, Javaheri M, Spee C, et al. Angiofibrotic response to vascular endothelial growth factor inhibition in diabetic retinal detachment: report no. 1. Arch Ophthalmol. 2012;130:1127–34.
Song Z, Sun M, Zhou F, Huang F, Qu J, Chen D. Increased intravitreous interleukin-18 correlated to vascular endothelial growth factor in patients with active proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2014;252:1229–34.
Song S, Yu X, Zhang P, Dai H. Increased levels of cytokines in the aqueous humor correlate with the severity of diabetic retinopathy. J Diabetes Complications. 2020;34:107641.
Spranger J, Bühnen J, Jansen V, Krieg M, Meyer-Schwickerath R, Blum WF. et al. Systemic levels contribute significantly to increased intraocularIGF-I, IGF-II and IGF-BP3 [correction of IFG-BP3] in proliferative diabeticretinopathy. Horm Metab Res.2000;32:196–200.
Spranger J, Hammes HP, Preissner KT, Schatz H, Pfeiffer AF. Release of the angiogenesis inhibitor angiostatin in patients with proliferative diabetic retinopathy: association with retinal photocoagulation. Diabetologia. 2000;43:1404–7.
Spranger J, Möhlig M, Osterhoff M, Bühnen J, Blum WF, Pfeiffer AF. Retinal photocoagulation does not influence intraocular levels of IGF-I, IGF-II and IGF-BP3 in proliferative diabetic retinopathy-evidence for combined treatment of PDR with somatostatin analogues and retinal photocoagulation? Horm Metab Res. 2001;33:312–6.
Spranger J, Osterhoff M, Reimann M, Möhlig M, Ristow M, Francis MK, et al. Loss of the antiangiogenic pigment epithelium-derived factor in patients with angiogenic eye disease. Diabetes. 2001;50:2641–5.
Srividya G, Jain M, Mahalakshmi K, Gayathri S, Raman R, Angayarkanni N. A novel and less invasive technique to assess cytokine profile of vitreous in patients of diabetic macular oedema. Eye (Lond). 2018;32:820–9.
Sun C, Zhang H, Jiang J, Li Y, Nie C, Gu J, et al. Angiogenic and inflammatory biomarker levels in aqueous humor and vitreous of neovascular glaucoma and proliferative diabetic retinopathy. Int Ophthalmol. 2020;40:467–75.
Suzuki Y, Suzuki K, Yokoi Y, Miyagawa Y, Metoki T, Nakazawa M. Effects of intravitreal injection of bevacizumab on inflammatory cytokines in the vitreous with proliferative diabetic retinopathy. Retina. 2014;34:165–71.
Suzuki Y, Suzuki K, Kudo T, Metoki T, Nakazawa M Level of Intravitreal IL-6 is Higher in Proliferative Diabetic Retinopathy Patients Without Preoperative Panretinal Photocoagulation (PRP) Than With Preoperative PRP. American Academy of Ophthalmology Annual Meeting Abstract, Chicago Illinois (2016).
Suzuki Y, Suzuki K, Kudo T, Metoki T, Nakazawa M. Level of vascular endothelial growth factor in the vitreous fluid of proliferative diabetic retinopathy patients and prognosis after vitrectomy. Ophthalmologica. 2016;236:133–8.
Suzuki Y, Yao T, Okumura K, Seko Y, Kitano S. Elevation of the vitreous body concentrations of oxidative stress-responsive apoptosis-inducing protein (ORAIP) in proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2019;257:1519–25.
Suzuki Y, Adachi K, Maeda N, Tanabu R, Kudo T, Nakazawa M. Proliferative diabetic retinopathy without preoperative pan-retinal photocoagulation is associated with higher levels of intravitreal IL-6 and postoperative inflammation. Int J Retin Vitreous. 2020;6:24.
Sydorova M, Lee MS. Vascular endothelial growth factor levels in vitreous and serum of patients with either proliferative diabetic retinopathy or proliferative vitreoretinopathy. Ophthalmic Res. 2005;37:188–90.
Takagi H, Watanabe D, Suzuma K, Kurimoto M, Suzuma I, Ohashi H, et al. Novel role of erythropoietin in proliferative diabetic retinopathy. Diabetes Res Clin Pr. 2007;77:S62–64.
Takahashi R, Sassa Y, Yoshida S, Saeki Y, Mitsutake T, Ishibashi T, et al. Increased concentrations of periostin in the vitreous of proliferative diabetic retinopathy patients with tractional retinal detachment. Invest Ophthalmol Vis Sci. 2014;55:1047.
Takahashi S, Adachi K, Suzuki Y, Maeno A, Nakazawa M. Profiles of inflammatory cytokines in the vitreous fluid from patients with rhegmatogenous retinal detachment and their correlations with clinical features. Biomed Res Int. 2016;2016:4256183.
Takeuchi M, Sato T, Shibata M, Taguchi M, Harimoto K, Sakurai Y Elevation of Th17 Cell-Related Cytokines in the Vitreous Fluids of Proliferative Diabetic Retinopathy. American Academy of Ophthalmology Annual Meeting Abstract, Chicago Illinois (2014).
Takeuchi M, Sato T, Tanaka A, Karasawa Y, Haimoto K, Sakurai Y, et al. Elevated levels of Th17-relevant cytokines in vitreous humor of patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2014;55:1872.
Takeuchi M, Sato T, Tanaka A, Muraoka T, Taguchi M, Sakurai Y, et al. Elevated levels of cytokines associated with Th2 and Th17 cells in vitreous fluid of proliferative diabetic retinopathy patients. PLoS One. 2015;10:e0137358.
Takeuchi M. Evaluation of T‐cell related cytokines in the vitreous of proliferative diabetic retinopathy. Acta Ophthalmologica. 2015;93:S255.
Takeuchi M, Taguchi M, Sato T, Karasawa K, Sakurai Y, Harimoto K, et al. Association of high-mobility group box-1 with th cell-related cytokines in the vitreous of ocular sarcoidosis patients. Invest Ophthalmol Vis Sci. 2017;58:528–37.
Takeuchi M, Sato T, Sakurai Y, Taguchi M, Harimoto K, Karasawa Y, et al. Association between aqueous humor and vitreous fluid levels of Th17 cell-related cytokines in patients with proliferative diabetic retinopathy. PLoS One. 2017;12:e0178230.
Takeuchi M, Sato T, Sakurai Y, Taguchi M, Harimoto K, Karasawa Y, et al. Association of Th17-cell related cytokines between aqueous humor and vitreous fluid in proliferative diabetic retinopathy patients. Invest Ophthalmol Vis Sci. 2017;58:5749.
Tamaki K, Usui-Ouchi A, Murakami A, Ebihara N. Fibrocytes and fibrovascular membrane formation in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57:4999–5005.
Tamaki K, Usui A, Ehihara N. Pentraxin3 in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58:5216.
Tanaka A, Sato T, Sakurai Y, Harimoto K, Takeuchi M. Association between vitreous level of IL-6 and factors involved in visual function. Invest Ophthalmol Vis Sci. 2015;56:5175.
Tao Y, Lu Q, Jiang YR, Qian J, Wang JY, Gao L, Jonas JB. Apelin in plasma and vitreous and in fibrovascular retinal membranes of patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51:4237–42.
Tashimo A, Mitamura Y, Nagai S, Nakamura Y, Ohtsuka K, Mizue Y, et al. Aqueous levels of macrophage migration inhibitory factor and monocyte chemotactic protein-1 in patients with diabetic retinopathy. Diabet Med. 2004;21:1292–7.
Teague GC, Nadakumar N, Ma J, Marra KV, Arroyo JG, Baldwin ME, et al. Development of biomarker equations based on multi-dimensional modeling of cytokine shifts in retinal diseases. Invest Ophthalmol Vis Sci. 2014;55:1919.
Tsai T, Kuehn S, Tsiampalis N, Vu MK, Kakkassery V, Stute G, et al. Anti-inflammatory cytokine and angiogenic factors levels in vitreous samples of diabetic retinopathy patients. PLoS One. 2018;13:e0194603.
Tsubota K, Wakabayashi Y, Ueda S, Usui Y, Suzuko J, Goto H. Effectiveness of prophylactic intravitreal bevacizumab injection in preventing complications after vitrectomy for proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54:198.
Tsubota K, Usui Y, Wakabayashi Y, Suzuki J, Ueda S, Goto H. Effectiveness of prophylactic intravitreal bevacizumab injection to proliferative diabetic retinopathy patients with elevated preoperative intraocular VEGF in preventing complications after vitrectomy. Clin Ophthalmol. 2019;13:1063–70.
Ucgun NI, Fikret CZ, Yildirim Z. Inflammation and diabetic macular edema. Ophthalmologica. 2014;232:220.
Ucgun NI, Zeki-Fikret C, Yildirim Z. Inflammation and diabetic retinopathy. Mol Vis. 2020;26:718–21.
Umeda N, Ozaki H, Hayashi H, Kondo H, Uchida H, Oshima K. Non-paralleled increase of hepatocyte growth factor and vascular endothelial growth factor in the eyes with angiogenic and nonangiogenic fibroproliferation. Ophthalmic Res. 2002;34:43–47.
Urbančič M, Petrovič D, Živin AM, Korošec P, Fležar M, Petrovič MG. Correlations between vitreous cytokine levels and inflammatory cells in fibrovascular membranes of patients with proliferative diabetic retinopathy. Mol Vis. 2020;26:472–82.
Van Geest RJ, Lesnik-Oberstein SY, Tan HS, Mura M, Goldschmeding R, Van Noorden CJ, et al. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br J Ophthalmol. 2012;96:587–90.
Van Geest RJ, Klaassen I, Lesnik-Oberstein SY, Tan HS, Mura M, Goldschmeding R, et al. Vitreous TIMP-1 levels associate with neovascularization and TGF-β2 levels but not with fibrosis in the clinical course of proliferative diabetic retinopathy. J Cell Commun Signal. 2013;7:1–9.
Vidhya S, Subbulakshmi C, Coral K, Pukhraj R, Sulochana KN. Measurement of adiponectin in vitreous and plasma of patients with proliferative diabetic retinopathy and its correlation with vascular endothelial growth factor, pigment epithelial derived factor and insulin like growth factor-1. Int J Pharm Biosci. 2013;4:993–1005.
Vingolo EM, Fragiotta S, Mafrici M, Cutini A, Marinelli C, Concistrè A, et al. Vitreous and plasma changes of endothelin-1, adrenomedullin and vascular endothelium growth factor in patients with proliferative diabetic retinopathy. Eur Rev Med Pharm Sci. 2017;21:662–8.
Vogels I, Van Geest RJ, Klaassen I, Lesnik-Oberstein SY, Goldschmeding R, Van Noorden CJF, et al. Vitreous Timp-1 levels associate with neovascularization and Tgf-2 levels in the clinical course of proliferative diabetic retinopathy. Eur J Ophthalmol. 2013;23:451.
Wakabayashi Y, Usui Y, Okunuki Y, Ueda S, Kimura K, Muramatsu D, et al. Intraocular VEGF level as a risk factor for postoperative complications after vitrectomy for proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2012;53:6403–10.
Wakabayashi Y, Usui Y, Tsubota K, Ueda S, Umazume K, Muramatsu D, et al. Persistent overproduction of intraocular vascular endothelial growth factor as a cause of late vitreous hemorrhage after vitrectomy for proliferative diabetic retinopathy. Retina. 2017;37:2317–25.
Wan W, Li Q, Zhang F, Zheng G, Lv Y, Wan G, Jin X. Serum and vitreous concentrations of omentin-1 in diabetic retinopathy. Dis Markers. 2015;2015:754312.
Wang X, Wang G, Wang Y. Intravitreous vascular endothelial growth factor and hypoxia-inducible factor 1a in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2009;148:883–9.
Wang J, Chen S, Jiang F, You C, Mao C, Yu J, et al. Vitreous and plasma VEGF levels as predictive factors in the progression of proliferative diabetic retinopathy after vitrectomy. PLoS One. 2014;9:e110531.
Wang Y, Gao S, Zhu Y, Shen X. Elevated activating transcription factor 4 and glucose-regulated 78 Kda protein levels correlate with inflammatory cytokines in the aqueous humor and vitreous of proliferative diabetic retinopathy. Curr Eye Res. 2017;42:1202–8.
Wang Y, Tao J, Yao Y. Prostaglandin E2 activates NLRP3 inflammasome in endothelial cells to promote diabetic retinopathy. Horm Metab Res. 2018;50:704–10.
Wang P, Wang WY, Zhang XD. Increased interleukin-26 expression in proliferative diabetic retinopathy. Int J Ophthalmol. 2019;12:1688–92.
Wang T, Li J, Xie R, Wang J, Zhang W, Jiang F, et al. Intraocular tumour necrosis factor ligand related molecule 1 A links disease progression of proliferative diabetic retinopathy after primary vitrectomy. Clin Exp Pharm Physiol. 2020;47:966–76.
Wang H, Lou H, Li Y, Ji F, Chen W, Lu Q, et al. Elevated vitreous Lipocalin-2 levels of patients with proliferative diabetic retinopathy. BMC Ophthalmol. 2020;20:260.
Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, Kita M, et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353:782–92.
Watanabe D, Suzuma K, Suzuma I, Ohashi H, Ojima T, Kurimoto M, et al. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2005;139:476–81.
Wei Q, Zhang T, Jiang R, Chang Q, Zhang Y, Huang X, et al. Vitreous fibronectin and fibrinogen expression increased in eyes with proliferative diabetic retinopathy after intravitreal Anti-VEGF therapy. Invest Ophthalmol Vis Sci. 2017;58:5783–91.
Wu H, Hwang DK, Song X, Tao Y. Association between aqueous cytokines and diabetic retinopathy stage. J Ophthalmol. 2017;2017:9402198.
Wu F, Phone A, Lamy R, Ma D, Laotaweerungsawat S, Chen Y, et al. Correlation of aqueous, vitreous, and plasma cytokine levels in patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2020;61:26.
Xing Q, Zhang G, Kang L, Wu J, Chen H, Liu G, et al. The suppression of Kallistatin on high-glucose-induced proliferation of retinal endothelial cells in diabetic retinopathy. Ophthalmic Res. 2017;57:141–9.
Xu Y, Cheng Q, Yang B, Yu S, Xu F, Lu L, et al. Increased sCD200 levels in vitreous of patients with proliferative diabetic retinopathy and its correlation with VEGF and proinflammatory cytokines. Invest Ophthalmol Vis Sci. 2015;56:6565–72.
Yabanoğlu DA, Tan CS, Kadayıfcılar S, Eldem B, Karahan S. Effect of adipose tissue-derived inflammatory and proangiogenic cytokines on proliferative diabetic retinopathy. Turk J Biochem. 2014;39:495–502.
Yan Y, Zhu L, Hong L, Deng J, Song Y, Chen X. The impact of ranibizumab on the level of intercellular adhesion molecule type 1 in the vitreous of eyes with proliferative diabetic retinopathy. Acta Ophthalmol. 2016;94:358–64.
Yan A, You H, Zhang X. Levels of Interleukin 27 and Interleukin 35 in the Serum and Vitreous of Patients with Proliferative Diabetic Retinopathy. Ocul Immunol Inflamm. 2018;26:273–9.
Yokoi M, Yamagishi SI, Takeuchi M, Ohgami K, Okamoto T, Saito W. Elevations of AGE and vascular endothelial growth factor with decreased total antioxidant status in the vitreous fluid of diabetic patients with retinopathy. Br J Ophthalmol. 2005;89:673–5.
Yokomizo H, Maeda Y, Park K, Clermont AC, Hernandez SL, Fickweiler W, et al. Retinol binding protein 3 is increased in the retina of patients with diabetes resistant to diabetic retinopathy. Sci Transl Med. 2019;11:eaau6627.
Yoshida S, Ishikawa K, Asato R, Arima M, Sassa Y, Yoshida A, et al. Increased expression of periostin in vitreous and fibrovascular membranes obtained from patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:5670–8.
Yoshida S, Nakama T, Ishikawa K, Arima M, Tachibana T, Nakao S, et al. Antiangiogenic shift in vitreous after vitrectomy in patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2012;53:6997–7003.
Yoshida S, Kobayashi Y, Nakama T, Zhou Y, Ishikawa K, Arita R, et al. Increased expression of M-CSF and IL-13 in vitreous of patients with proliferative diabetic retinopathy: implications for M2 macrophage-involving fibrovascular membrane formation. Br J Ophthalmol. 2015;99:629–34.
Yoshida S, Kubo Y, Kobayashi Y, Zhou Y, Nakama T, Yamaguchi M, et al. Increased vitreous concentrations of MCP-1 and IL-6 after vitrectomy in patients with proliferative diabetic retinopathy: possible association with postoperative macular oedema. Br J Ophthalmol. 2015;99:960–6.
Yoshida S, Kobayashi Y, Nakao S, Sassa Y, Hisatomi T, Ikeda Y, et al. Differential association of elevated inflammatory cytokines with postoperative fibrous proliferation and neovascularization after unsuccessful vitrectomy in eyes with proliferative diabetic retinopathy. Clin Ophthalmol. 2017;11:1697–705.
Yoshida S, Murata M, Noda K, Matsuda T, Saito M, Saito W, et al. Proteolytic cleavage of vascular adhesion protein-1 induced by vascular endothelial growth factor in retinal capillary endothelial cells. Jpn J Ophthalmol. 2018;62:256–64.
Yoshimura T, Sonoda KH, Sugahara M, Mochizuki Y, Enaida H, Oshima Y, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009;4:e8158.
Yu G, Ji X, Jin J, Bu S. Association of serum and vitreous concentrations of osteoprotegerin with diabetic retinopathy. Ann Clin Biochem. 2015;52:232–6.
Yu Y, Zhang J, Zhu R, Zhao R, Chen J, Jin J, et al. The profile of angiogenic factors in vitreous humor of the patients with proliferative diabetic retinopathy. Curr Mol Med. 2017;17:280–6.
Yuuki T, Kanda T, Kimura Y, Kotajima N, Tamura J, Kobayashi I, et al. Inflammatory cytokines in vitreous fluid and serum of patients with diabetic vitreoretinopathy. J Diabetes Complications. 2001;15:257–9.
Zeng Y, Cao D, Yu H, Hu Y, He M, Yang D, et al. Comprehensive analysis of vitreous humor chemokines in type 2 diabetic patients with and without diabetic retinopathy. Acta Diabetol. 2019;56:797–805.
Zhang X, Wu J, Wu C, Bian AL, Geng S, Dai RP. Comparison of aqueous humor levels of PlGF and VEGF in proliferative diabetic retinopathy before and after intravitreal conbercept injection. Diabetes Res Clin Pr. 2020;162:108083.
Zhang H, Liang L, Huang R, Wu P, He L. Comparison of inflammatory cytokines levels in the aqueous humor with diabetic retinopathy. Int Ophthalmol. 2020;40:2763–9.
Zhao M, Bai Y, Xie W, Shi X, Li F, Yang F, et al. Interleukin-1β Level Is Increased in Vitreous of Patients with Neovascular Age-Related Macular Degeneration (nAMD) and Polypoidal Choroidal Vasculopathy (PCV). PLoS One. 2015;10:e0125150.
Zhao M, Hu Y, Yu Y, Lin Q, Yang J, Su SB, et al. Involvement of IL-37 in the pathogenesis of proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57:2955–62.
Zheng B, Li T, Chen H, Xu X, Zheng Z. Correlation between ficolin-3 and vascular endothelial growth factor-to-pigment epithelium-derived factor ratio in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 2011;152:1039–43.
Zhou J, Wang S, Xia X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res. 2012;37:416–20.
Zhou W, Wang H, Yu W, Xie W, Zhao M, Huang L, et al. The expression of the Slit-Robo signal in the retina of diabetic rats and the vitreous or fibrovascular retinal membranes of patients with proliferative diabetic retinopathy. PLoS One. 2017;12:e0185795.
Zhou J, Liu Z, Chen M, Luo ZH, Li YQ, Qi GY, et al. Concentrations of VEGF and PlGF Decrease in Eyes After Intravitreal Conbercept Injection. Diabetes Ther. 2018;9:2393–8.
Zhu X, Bai Y, Yu W, Pan C, Jin E, Song D, et al. The effects of pleiotrophin in proliferative diabetic retinopathy. PLoS One. 2015;10:e0115523.
Brucker AJ, Qin H, Antoszyk AN, Beck RW, Bressler NM, Browning DJ, et al. Observational study of the development of diabetic macular edema following panretinal (scatter) photocoagulation given in 1 or 4 sittings. Arch Ophthalmol. 2009;127:132–40.
Googe J, Brucker AJ, Bressler NM, Qin H, Aiello LP, Antoszyk A, et al. Randomized trial evaluating short-term effects of intravitreal ranibizumab or triamcinolone acetonide on macular edema after focal/grid laser for diabetic macular edema in eyes also receiving panretinal photocoagulation. Retina. 2011;31:1009–27.
Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314:2137–46.
Wu J, Zhong Y, Yue S, Yang K, Zhang G, Chen L, et al. Aqueous humor mediator and cytokine aberrations in diabetic retinopathy and diabetic macular edema: a systematic review and meta-analysis. Dis Markers. 2019;2019:6928524.
Yao Y, Li R, Du J, Long L, Li X, Luo N. Interleukin-6 and diabetic retinopathy: a systematic review and meta-analysis. Curr Eye Res. 2019;44:564–74.
Cai J, Kehoe O, Smith GM, Hykin P, Boulton ME. The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:2163–71.
Rangasamy S, Srinivasan R, Maestas J, McGuire PG, Das A. A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:3784–91.
Li BY, Tan W, Zou JL, He Y, Yoshida S, Jiang B, et al. Role of interferons in diabetic retinopathy. World J Diabetes. 2021;12:939–53.
Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem 270, 27348-57 (1995z).
Campochiaro PA, Hackett SF, Vinores SA, Freund J, Csaky C, LaRochelle W, et al. Platelet-derived growth factor is an autocrine growth stimulator in retinal pigmented epithelial cells. J Cell Sci. 1994;107:2459–69.
Mori K, Gehlbach P, Ando A, Dyer G, Lipinsky E, Chaudhry AG, et al. Retina-specific expression of PDGF-B versus PDGF-A: vascular versus nonvascular proliferative retinopathy. Invest Ophthalmol Vis Sci. 2002;43:2001–6.
Melter M, Reinders ME, Sho M, Pal S, Geehan C, Denton MD, et al. Ligation of CD40 induces the expression of vascular endothelial growth factor by endothelial cells and monocytes and promotes angiogenesis in vivo. Blood. 2000;96:3801–8.
Minaker SA, Mason RH, Bamakrid M, Lee Y, Muni RH. Changes in aqueous and vitreous inflammatory cytokine levels in retinal vein occlusion: a systematic review and meta-analysis. J Vitreoretin Dis. 2020;4:36–64.
Minaker SA, Mason RH, Lahaie Luna G, Bapat P, Muni RH. Changes in aqueous and vitreous inflammatory cytokine levels in neovascular age-related macular degeneration: a systematic review and meta-analysis. Acta Ophthalmol. 2021;99:134–55.
Whitehead M, Wickremasinghe S, Osborne A, Van Wijngaarden P, Martin KR. Diabetic retinopathy: a complex pathophysiology requiring novel therapeutic strategies. Expert Opin Biol Ther. 2018;18:1257–70.
Stellmach V, Crawford SE, Zhou W, Bouck N. Prevention of ischemia-induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium-derived factor. Proc Natl Acad Sci USA. 2001;98:2593–7.
Liu H, Ren JG, Cooper WL, Hawkins CE, Cowan MR, Tong PY. Identification of the antivasopermeability effect of pigment epithelium-derived factor and its active site. Proc Natl Acad Sci USA. 2004;101:6605–10.
Zhang SX, Wang JJ, Gao G, Parke K, Ma JX. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006;37:1–12.
Gao G, Li Y, Zhang D, Gee S, Crosson C, Ma J. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett. 2001;489:270–6.
Bouck N. PEDF: anti-angiogenic guardian of ocular function. Trends Mol Med. 2002;8:330–4.
Chatzigeorgiou A, Harokopos V, Mylona-Karagianni C, Tsouvalas E, Aidinis V, Kamper EF. The pattern of inflammatory/anti-inflammatory cytokines and chemokines in type 1 diabetic patients over time. Ann Med. 2010;42:426–38.
Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–72.
Granger JP. An emerging role for inflammatory cytokines in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H923–924.
Feingold KR, Grunfeld C. Role of cytokines in inducing hyperlipidemia. Diabetes. 1992;41:97–101.
Meacock WR, Spalton DJ, Stanford MR. Role of cytokines in the pathogenesis of posterior capsule opacification. Br J Ophthalmol. 2000;84:332–6.
Zandi S, Tappeiner C, Pfister IB, Despont A, Rieben R, Garweg JG. Vitreal cytokine profile differences between eyes with epiretinal membranes or macular holes. Invest Ophthalmol Vis Sci. 2016;57:6320–6.
Author information
Authors and Affiliations
Contributions
RHM (or Mason) had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. RHM provided study concept. SAM designed the study. All authors acquired the data. RHM, SAM, and RHM analysed or interpreted the data. RHM and SAM conducted the statistical analysis. RHM, SAM, GLL and RHM drafted the manuscript. All authors critically revised the manuscript for important intellectual content. RHM supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This systematic review and meta-analysis did not involve any participants, and thus ethical approval was not necessary.
Informed consent
There were no direct participants in our study, and thus informed consent was not necessary.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mason, R.H., Minaker, S.A., Lahaie Luna, G. et al. Changes in aqueous and vitreous inflammatory cytokine levels in proliferative diabetic retinopathy: a systematic review and meta-analysis. Eye (2022). https://doi.org/10.1038/s41433-022-02127-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41433-022-02127-x
This article is cited by
-
Translating discoveries as novel biomarkers and interventions in ophthalmology
Journal of Translational Medicine (2023)
-
Comment on: Changes in aqueous and vitreous inflammatory cytokine levels in proliferative diabetic retinopathy: a systematic review and meta-analysis
Eye (2023)
-
Potential Protective Function of Adiponectin in Diabetic Retinopathy
Ophthalmology and Therapy (2023)
-
Vitreous protein networks around ANG2 and VEGF in proliferative diabetic retinopathy and the differential effects of aflibercept versus bevacizumab pre-treatment
Scientific Reports (2022)