Abstract
Purpose
To report outcomes of deep sclerectomy (DS) with intraoperative mitomycin C (MMC) application in eyes with previous failed glaucoma surgery (GS) and/or cataract extraction (CE).
Patients and methods
Single-surgeon case series of 82 eyes of 82 patients undergoing DS with MMC. The patients had previous CE with IOL and/or conjunctival GS and treated intraocular pressure (IOP) >18 mm Hg. MMC (0.2 mg/ml) was applied for 2–3 min before scleral flap dissection. Complete success was defined as IOP between 6 and 21 mm Hg or a reduction of 20% from baseline without medications. Reoperation for glaucoma or related complications, or loss of light perception vision was considered as failure.
Results
Mean follow-up was 57.7±22.4 months with 78% of patients completing the 3-year follow-up. Mean IOP decreased from 24.0 mm Hg (22.3–25.6, 95% confidence intervals) to 13.4 mm Hg (12.0–14.2) at 3 years after surgery (P<0.001). There was a significant decrease in the number of glaucoma medications from 2.0±1 preoperatively, to 0.3±0.7, 3 years after surgery. Kaplan–Meier cumulative success rates were 85.6% at 1 year, 80.0% at 2 years, and 76% at 3 years. At 3 years, IOP was maintained <19 and 15 mm Hg in 83 and 70% of eyes, respectively. Fourteen eyes (17.1%) had complications. Delayed hypotony (IOP <6 mm Hg) was the commonest complication in five eyes (6.1%).
Conclusion
DS with MMC appears to be a safe and effective surgical procedure for eyes with previous intraocular surgery.
Similar content being viewed by others
Introduction
Non-penetrating glaucoma surgery (NPGS) like viscocanalostomy1 and deep sclerectomy (DS) were popularised in the 1990s as safer alternatives to trabeculectomy.2 The essential differences between NPGS and trabeculectomy are that the surgical procedure entails the creation of a filtration membrane, the trabeculo-Descemet's membrane (TDM) rather than a sclerostomy and the excision of the inner scleral flap creates a subscleral lake. Outflow pathways remain unclear but increased aqueous flow through the Schlemm's canal, intrascleral ‘bleb’ and subsequent suprachoroidal flow and subconjunctival flow with ‘bleb’ formation have all been proposed.3, 4, 5, 6, 7, 8, 9 DS is the basic NPGS procedure. A spacer device is often placed on the scleral bed to prevent adhesion of the scleral flap to the bed and help form a subscleral lake and subconjunctival filtration bleb.2 The comparative efficacy of the various types of NPGS to trabeculectomy remains a subject of some debate but most reports agree that NPGS has a lower rate of complications than trabeculectomy.10, 11, 12, 13, 14, 15 DS has been shown to be equivalent to trabeculectomy in lowering the intraocular pressure (IOP) in some recent publications.11, 16, 17 Excellent long-term results have been reported with DS as the primary surgery for open-angle glaucoma.18, 19, 20 The IOP after DS can be further lowered by Nd:YAG laser goniopuncture (LGP) of the TDM.21, 22 Like with trabeculectomy, intraoperative mitomycin C (MMC) application results in lower IOPs in the long term23, 24 and bleb rejuvenation techniques like needle revision can be used.25 DS with MMC has been reported to be a safe and effective procedure for eyes with failed trabeculectomy.26, 27
There has been a lack of consensus among glaucoma surgeons as to the best surgical option in eyes at a high-risk-to-failure, such as previous intraocular surgery. The Tube versus Trabeculectomy (TVT) trial was designed to prospectively assess trabeculectomy and Bearveldt tube implant (BGI) surgery, both augmented with MMC, in eyes with previous cataract surgery and/or failed trabeculectomy.28, 29, 30, 31 The study suggests that better IOP outcomes are achieved after BGI compared with trabeculectomy at 3 years. However, eyes with tube surgery may be prone to more serious long-term complications like corneal decompensation and diplopia. In this report, we have sought to examine the outcomes of DS with intraoperative MMC in combination with the full complement of postoperative procedures in eyes with either previous cataract extraction (CE) or glaucoma surgery (GS) based on the TVT trial criteria of success.
Materials and methods
Patients were identified from a database of all patients undergoing GS by one surgeon (NA) between August 2001 and March 2008. Data entry was completed at the time of surgery and contemporaneously at each postoperative visit. Inclusion and exclusion criteria and outcome measures were similar to those of the TVT trial. Primary open-angle glaucoma patients older than 20 years with previous CE with IOL implantation and/or conjunctival GS (mainly trabeculectomy) were included. Clear corneal incisions were done for all cataract surgeries, phaco-emulsification or extracapsular technique. Eyes with aphakia and uveitic glaucoma were excluded. Eyes with previous vitreo-retinal surgery were included (excluded from TVT trial). In patients where both eyes were eligible, the eye operated on first was included. Data extracted from database included, Snellen visual acuity (VA), IOP, postoperative complications, subsequent procedures including reoperation for glaucoma, use of supplemental medical therapy.
Peribulbar or subtenon's regional anaesthesia was used for most cases and general anaesthesia in some cases according to the patient's preference. A 6-0 vicryl traction suture was used to infraduct the globe and a fornix-based conjunctival flap was fashioned. MMC was applied at a dose of 0.2 mg/ml for 2–3 min on four PVA sponge fragments placed under the conjunctival flap. The surgical area was then irrigated with 10 ml Balance salt solution. A 5 × 5 mm superficial scleral flap, hinged at the limbus, was created to approximately one-third scleral depth and reflected 1 mm into the clear cornea. Within the bed of the superficial flap, a 90% depth scleral flap was fashioned and the ends of Schlemm's canal cut. Dissection of the deep scleral flap was continued into clear cornea to expose a trabeculo-descemetic window (TDM) of ∼3–4 mm width. Juxtacanalicular trabecular meshwork was peeled using blunt-tipped capsulorhexis forceps. The deep scleral flap was then excised. Topical 1% fluorescein was applied to assess egress of aqueous humour across the TDM and stain residual tissue to be removed in the dissection. A 3.5-mm reticulated hyaluronic acid absorbable spacer implant (SKGel Corneal Laboratories, Paris, France) was placed under the superficial scleral flap, which was then sutured with 10-0 nylon. Porcine collagen implants (Aquaflow, STAAR, Nidau, Switzerland) were used in later cases as SKGel Implant manufacture had stopped. The outer scleral flap and conjunctiva were then closed with 10-0 nylon-interrupted sutures. If a microperforation occurred without prolapse of iris, then the surgery was continued as described above. If a macroperforation occurred then a peripheral iridotomy was made in the presenting iris and the procedure was converted into trabeculectomy. Postoperatively patients received prednisolone acetate 1% drops six times a day continued for a minimum of 8 weeks. Patients were followed up at day 1 and then at weeks 1 and 6, or more frequently if required. Thereafter, follow-up was determined by clinical need. Where there was an elevation of the IOP at any stage, Nd:Yag LGP was performed with a CGAL (Haag-Streit, Koeniz, Switzerland) contact gonioscopy lens. Needle revision with 5-Fluorouracil (5-FU) or MMC was performed on the slit-lamp biomicroscope if the IOP was still elevated. Argon and Nd:YAG iridoplasty were done either prophylactically to avoid iris prolapse into the goniopuncture or to remove iris incarcerated in the goniopuncture. These postoperative interventions were recorded contemporaneously as part of data collection. The techniques for LGP and iridoplasty are described in detail in a previous publication.21
Definitions were derived from the TVT trial.28 Reoperation for glaucoma or a complication was defined as additional surgery requiring a return to the operating theatre. Interventions performed in the outpatient's clinic setting, such as needling procedures or LGP, were not considered reoperations. Serious complications were defined as surgical complications associated with loss of two or more lines of Snellen VA for >6 months and/or reoperation to manage the complication. Eyes that tested Seidel positive within the first month of follow-up were classified as wound leaks, and those occurring after 1 month were categorized as bleb leaks. Patients who underwent additional GS were censored from analysis of complications after the reoperation for glaucoma. Cataracts were considered to have progressed if there was loss of two or more lines of Snellen VA that was attributed to cataract, or if cataract surgery was performed.
Success criteria were similar to that of the TVT trial.32 Failure was defined as IOP >21 mm Hg or <20% reduction below baseline on two consecutive follow-up visits after 3 months. IOP <5 mm Hg on two consecutive follow-up visits after 3 months, reoperation for glaucoma, or loss of light perception vision were considered as failure. Eyes that met these criteria and were not on any supplemental medications were defined as complete success. Eyes that had not failed but required supplemental medical therapy were defined as qualified successes. Needle revision and LGP were not considered as failure.
The time to failure was defined either as the time from surgical treatment to reoperation for glaucoma or as the time from surgery to the first visit in which the patient had hypotony (IOP 5 mm Hg) or inadequately reduced IOP (IOP 21 mm Hg or not reduced by 20% below baseline). This implies that if a patient had an unsuccessful LGP or needle revision, failure was considered to have occurred on the visit when the decision to do these procedures was taken. IOP changes over time and comparison of IOPs between groups was done by analysis of variance and of glaucoma medications by the Kruskal–Wallis test. Univariate comparisons between treatment groups were performed using the two-sided Student's t-test for continuous variables and the Yates corrected χ2 test or the Fisher's exact test. Treatment comparisons of time to failure were assessed with Kaplan–Meier survival analysis, log-rank test, and Cox's regression analyses. All tests were two-tailed, and P-values <0.05 were taken to be significant. Data presentation adheres to the guidelines suggested by Jabs for clinical case series.33
Results
Patient demographics are shown in Table 1. Eighty-two patients fulfilled the study criteria and were included in the analyses. Of these, 30 had previous GS, 17 had both cataract surgery and GS, and 34 had previous cataract surgery. The details are shown in Table 2. Grouping was done as in the TVT study and eyes with both cataract surgery and GS were included in the GS group. Thirty-seven eyes (45.1%) had two or more surgeries before the DS and only six of these eyes were in the CE group. The GS group had significantly higher number of previous intraocular surgeries (1.9±0.7) compared with the CE group (1.2±0.5, P<0.00). The mean follow-up duration was 57.7±22.4 months. All patients completed the first year's follow-up, 75 (91.5%) completed the second year's follow-up, and 64 (78%) the third year's follow-up. Twenty patients (24.2%) died during the observation period and two (2.4%) became too ill to attend the clinic.
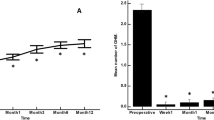
There was a significant drop in IOP from baseline to 5 years after surgery. Mean IOP decreased from 24.0 mm Hg (22.3–25.6, 95% confidence intervals (CIs)) to 13.4 mm Hg (12.0–14.2, 95% CI) at 3 years after surgery (P<0.001). IOP changes after surgery are shown in Figure 1. There was a significant decrease in the number of glaucoma medications from 2.0±1 (SDS) preoperatively, to 0.3±0.7, 3 years after surgery. Table 3 shows the IOP and medications at baseline to 5 years after the surgery. No statistical difference was noted at any time between the groups in the number of medications and IOP. At last follow-up 18 eyes (22%), 13 from the GS group and 5 from the CE group were on glaucoma medications (P=0.2).
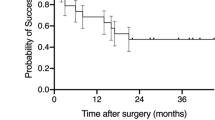
The complete success rates by Kaplan–Meier survival analysis were 85.4% at 1 year, 80.0% at 2 years, and 76% at 3 years (Figure 2). Table 4 summarizes cumulative success rates by different criteria at 3 years after DS. Figure 3 depicts the comparative outcome plots between the two groups for success defined as IOP <18 and 15 mm Hg. A Cox's regression analyses model suggested a significantly higher failure rate for eyes with two or more previous intraocular surgeries (hazards ratio, 4.6; 95% CI, 1.6–11.5; P=0.004) and surgeries complicated by intraoperative perforation of the TDM (HR 3.9, 95% CI, 1.2–13.2; P=0.03) after adjusting for age, sex, laterality, baseline IOP, previous exposure to MMC or 5-FU, MMC dose and duration of application, and use of or type of spacer device. Univariate comparison by the log-rank tests confirmed significantly lower success rates in eyes with more than two previous intraocular surgeries (P=0.001) and with intraoperative perforation of the TDM (P=0.01).
Kaplan–Meier plots of the cumulative probability of success defining adequate IOP reduction as IOP >17 mm Hg (left) or IOP >14 mm Hg (right). Patients with persistent hypotony, reoperation for glaucoma, and loss of light perception vision are classified as failures. CE, previous cataract surgery; GS, previous glaucoma surgery.
Treatment failures over the follow-up period were not uncommon and 27 eyes (32.9%) failed. The most common cause was inadequate IOP reduction in 19 eyes (23.2%) and the highest number of failures was in the subgroup of eyes with previous cataract surgery and GS (13 eyes). Repeat procedure was cause of failure in two eyes (3.7%) and hypotony in five eyes (6.1%). Eyes in GS group were more likely to be treatment failures than those in CE group (P=0.008). Kaplan–Meier survival plots showed that eyes with both previous cataract surgery and GS fared worse than eyes with either previous cataract surgery or GS alone (P=0.03; Figure 4).
Intraoperative perforation of the TDM was noted in eight eyes (9.8%). All perforations occurred in pseudophakic eyes and there was no iris prolapse. Therefore, peripheral iridectomy was not required in any of these cases. Intraoperative suprachoroidal haemorrhage after a perforation was seen in an eye, which also had a corneal graft. The haemorrhage was drained partially through two inferior L-shaped choroidotomies. The eventual outcome was favourable, with good IOP control and no change in VA from baseline until the patient's death 2 years later.
Early and late complications are presented in Table 5. Fourteen eyes (17.1%) had complications. Delayed hypotony was the commonest complication in five eyes (6.1%) and three of these had macular changes. All eyes with hypotony were from the GS group. Details and management of each case is presented in Table 6. Corneal oedema was noted after a scleral patch graft procedure for hypotony in one eye. In another eye, there was corneal oedema after reactivation of herpes simplex keratitis. The patient also had subconjunctival MMC of 0.02 mg before a needle revision procedure, which may have contributed to corneal endothelial cell loss. Serious complications resulting in reoperation and/or loss of two or more Snellen VA lines occurred in six eyes (7.3%). Three eyes each had surgery for bleb dysaesthesia and delayed ocular hypotony.
Procedures after DS are presented in Table 7. The Kaplan–Meier probability of performing LGP was 57.3% at 1 year and 63.6% at 3 years and there was no significant difference between the groups (P=0.34, log-rank test). Argon laser iridoplasty was done in 10 (12.2%) eyes. The indication was iris contact with the TDM in three, iris plugging the goniopuncture in one, and prophylactically in six eyes. The latter were eyes with either a narrow entry into the angle (Shaeffer's grades 1 and 2) or antero-posteriorly narrow TDM windows with a posterior goniopuncture and where iris incarceration was anticipated. LGP was effective in lowering IOP by 20% and below 21 mm Hg without medications or needle revision in 19 eyes of the 53 eyes over the follow-up period. Needle revision of the failing filtration bleb was performed in 26 eyes (31.7%) and was successful in lowering IOP by the same criteria in 12 of these eyes. Needle revision with MMC was repeated in four eyes and with 5-FU in two eyes. Corneal decompensation occurred in one eye, which underwent two needle revisions (as reported in previous paragraph). Revision of the failing DS by reopening the surgical site, further MMC application, and a peripheral iridectomy was done in three eyes (3.7%). Repeat penetrating keratoplasty was done on an eye with a corneal graft, which had failed before eye had undergone DS.
A reduction of VA by two or more Snellen lines from baseline at the 6-month follow-up visit or after was noted in 18 eyes (22.0%) by last follow-up. The commonest cause was progression of age-related macular degeneration in six eyes. Progression of glaucomatous optic neuropathy was the cause in two eyes, including one patient with a preoperative visual field mean deviation of more than −30 dB. The patient's VA decreased from 6/18 to perception of hand movements only, 3 years after surgery. Hypotony with associated maculopathy was the cause of reduced VA in two eyes. Progression of cataract, diabetic macular oedema, macular hole, corneal oedema, severe dry eye, dementia, and no refraction were the cause of VA reduction in one eye each.
Discussion
The final route for aqueous outflow after DS has been a subject of research and controversy. Manifestly, it is a bleb-dependent NPGS procedure. A recent experimental study in porcine eyes suggests that the outflow routes could be all three—the subconjunctival pathway, the functional deep scleral lake, and the opening of the Schlemm's canal.4 However, as shown by ultrasound biomicroscopy (UBM) studies, there is a poor correlation between the intrascleral lake dimensions and IOP control. It is likely that in most eyes after DS, the subconjunctival outflow route is essential for lowering IOP. Therefore, techniques that promote bleb survival in trabeculectomy may be applicable to DS. A prospective randomized trial has shown that intraoperative MMC application during DS improves long-term IOP control.23 A case–control study showed MMC use and a low IOP on the first postoperative day (an indicator of adequate deep scleral flap dissection) were significantly related to maintaining an IOP <18 mm Hg, 2 years after DS.24 While there is no reported study of comparison in morphology between DS and trabeculectomy blebs, we have reported that anterior limbal MMC application results in a high frequency of avascular and thin-walled blebs in both DS and trabeculectomy.34 In trabeculectomy, intensive postoperative management may lead to lower IOPs in the long term.35 Our postoperative regime for DS, besides topical steroids, consists of subconjunctival 5-FU injections for failing blebs, LGP and needle revision with subconjunctival MMC or 5-FU. Interestingly, no eye in this study was given postoperative subconjunctival 5-FU. A UBM study has demonstrated that lower IOP after DS is correlated with the thickness of the TDM.5 Nd:YAG LGP can be done in the early postoperative period if there was difficultly in deep dissection or if the IOP is raised significantly in the early postoperative period. We now tend to delay LGP to after 3 months, after topical steroid withdrawal. In this case series, LGP was done within the first 3 months in 50% of cases. Needle revision was done after unsuccessful LGP and 48% of the eyes that had LGP went on to have needle revision. In effect this probably converts the DS to a partial-thickness fistula, not unlike a trabeculectomy without a peripheral iridectomy. These postoperative manipulations may have contributed to improving the success rates of DS in 37.8% of eyes in this case series.
The TVT trial has provided a benchmark to evaluate glaucoma procedures in high-risk-to-failure eyes.28, 29, 30, 31 The mean IOP was ∼13 mm Hg at 3 years in both, the trabeculectomy and tube groups, similar to that of DS in our study. Complete (medication-free) success rates at 3 years were much lower than our study. They were 28 and 33% for the tube and trabeculectomy groups, respectively, compared with 74% for DS. Failure rates (100% minus partial and complete success rates) were significantly higher at 30.7% in the trabeculectomy compared with 15.1% at 3 years in the tube group. Using the same criteria for failure as the TVT trial, the failure rates of DS were 10.0% at 3 years. The mean number of medication for DS at 0.3±0.7 in 3 years compared with 1.3±1.3 for the tube and 1.0±1.5 for the trabeculectomy group in the TVT study. This is probably due to the aforementioned positive effect on IOP of LGP and needle revision on DS. Success rates may also have been enhanced by the fact this is a single-surgeon series. Cataract surgery in the United Kingdom (and in our centres) is performed through corneal sections. In contrast, in the United States, most surgeons use the posterior limbal section with conjunctival flaps. It is possible that this makes previous cataract surgery a more significant risk factor in the TVT trial than in our study.
The complication rate in the present study compares favourably with the TVT study in terms of both overall complication rate and serious complications.31 This may be interpreted with some caution because of the retrospective nature of our study. On the other hand, mean follow-up was about 5 years, increasing the probability of observing delayed complications like bleb leak, bleb-related infections and dysaesthesia and ocular hypotony. The high incidence of hypotony (6.1%) after DS in this study was unexpected. We have previously reported hypotony with maculopathy in 1.5% of eyes after primary phakic DS augmented with MMC.25 Hypotony was observed only after a postoperative procedure to lower IOP was performed in all except one eye (Table 6). The outer scleral flap after DS is very thin offers minimal resistance to aqueous outflow. MMC and cautery may cause further thinning of the scleral flap. In some eyes with minimal subconjunctival resistance, LGP or needle revision with antimetabolites may precipitate hypotony. To avoid hypotony, we have made some technical modifications like increasing the thickness of the outer scleral flap, minimising cautery, and avoiding MMC contact with the flap. LGP is delayed for a few months after surgery and not done in eyes with an IOP of <18 mm Hg. Subconjunctival and repeat MMC use is altogether avoided. MMC is not used in patients over the age of 80 years, regardless of risk status. Another indicator of the safety of the procedure is the number of procedures required for complications. In this study, 8 (9.8%) eyes required 10 procedures to deal with complications such as bleb dysaesthesia, hypotony, and delayed bleb leak. In the TVT study, 15% of eyes the tube group and 9% of eyes in the trabeculectomy group had procedures for complications.
DS may have some advantages over trabeculectomy in high-risk-to-failure eyes. Trabeculectomy is associated with an increase in anterior chamber (AC) inflammation, which may compromise bleb survival.36 AC activity, as measured by a flare-meter, is significantly lower after DS than trabeculectomy and this may be advantageous for bleb survival in high-risk eyes.37 Excessive flow in the early postoperative period may result in a number of complications with trabeculectomy. The presence of the TDM in DS ensures a predictable steady flow of aqueous and a low IOP in the early postoperative period, a favourable prognostic sign. After DS, shallow AC, hyphema, and AC inflammation are infrequently seen. There are some disadvantages of DS, the most important in this setting being the technical difficulty of performing the procedure in the nasal or temporal quadrant where the limbus is narrow, making it more difficult to dissect a wide TDM window. The long-learning curve of DS is a serious limitation.
This study, in common with other retrospective studies, has several limitations. Observer bias is inherent in study design. A significant proportion of the patients, about 30%, were lost to follow-up. The number of early complications may have been underestimated. The report includes cases over an 8-year period and DS has a long-learning curve. In addition, the postoperative management has been poorly defined in the literature and it evolved during the course of the study. The patients in this study were almost exclusively of Caucasian origin, limiting the extent to which comparisons with the TVT trial can be made. The glaucoma procedure of choice in the setting of previous failed GS remains controversial and an unresolved issue. While the TVT generally points towards the superiority of tube shunt surgery in the event of tube failure options for further surgery are limited.38 Another researcher has pointed out that the outcomes of trabeculectomy in the TVT study are significantly inferior to those reported from their institution.39 The complication rates of trabeculectomy are quite similar to those reported by our group on surgical reopening of the scleral flap with MMC application in eyes with failed trabeculectomy. We had concluded that the complication rates were too high with surgical reopening of the failed filter and it may be preferable to perform repeat surgery at a fresh site.40 Perhaps, the choice of surgery will always be dictated by the training and individual skills of the surgeon. In conclusion, this report shows that DS has IOP outcomes similar to trabeculectomy and tube surgery in eyes with previous cataract surgery and/or GS.

References
Stegmann R, Pienaar A, Miller D . Viscocanalostomy for open-angle glaucoma in black African patients. J Cataract Refract Surg 1999; 25: 316–322.
Sanchez E, Schnyder CC, Sickenberg M, Chiou AG, Hediguer SE, Mermoud A . Deep sclerectomy: results with and without collagen implant. Int Ophthalmol 1996; 20: 157–162.
Lei J, Sun N, Zhao X, Kang Q, Chen L, Fan X . Morphologic study of the drainage pathway using a tracer after a bypass filtering procedure in rabbit eyes. Ophthalmic Surg Lasers Imaging 2011; 42: 254–262.
Xu W, Yao K, Wu W, Li Z, Ye P . Change in outflow pathway of porcine eyes in vitro by nonpenetrating filtering surgery. Can J Ophthalmol 2010; 45: 632–636.
Aptel F, Dumas S, Denis P . Ultrasound biomicroscopy and optical coherence tomography imaging of filtering blebs after deep sclerectomy with new collagen implant. Eur J Ophthalmol 2009; 19: 223–230.
Delarive T, Rossier A, Rossier S, Ravinet E, Shaarawy T, Mermoud A . Aqueous dynamic and histological findings after deep sclerectomy with collagen implant in an animal model. Br J Ophthalmol 2003; 87: 1340–1344.
Johnson DH, Johnson M . How does nonpenetrating glaucoma surgery work? Aqueous outflow resistance and glaucoma surgery. J Glaucoma 2001; 10: 55–67.
Roters S, Luke C, Jonescu-Cuypers CP, Engels BF, Jacobi PC, Konen W et al. Ultrasound biomicroscopy and its value in predicting the long term outcome of viscocanalostomy. Br J Ophthalmol 2002; 86: 997–1001.
Kazakova D, Roters S, Schnyder CC, Achache F, Jonescu-Cuypers C, Mermoud A et al. Ultrasound biomicroscopy images: long-term results after deep sclerectomy with collagen implant. Graefes Arch Clin Exp Ophthalmol 2002; 240: 918–923.
Carassa RG, Bettin P, Fiori M, Brancato R . Viscocanalostomy versus trabeculectomy in white adults affected by open-angle glaucoma: a 2-year randomized, controlled trial. Ophthalmology 2003; 110: 882–887.
Cillino S, Di PF, Casuccio A, Cillino G, Lodato G . Deep sclerectomy versus trabeculectomy with low-dosage mitomycin C: four-year follow-up. Ophthalmologica 2008; 222: 81–87.
O'Brart DP, Rowlands E, Islam N, Noury AM . A randomised, prospective study comparing trabeculectomy augmented with antimetabolites with a viscocanalostomy technique for the management of open angle glaucoma uncontrolled by medical therapy. Br J Ophthalmol 2002; 86: 748–754.
Yarangumeli A, Gureser S, Koz OG, Elhan AH, Kural G . Viscocanalostomy versus trabeculectomy in patients with bilateral high-tension glaucoma. Int Ophthalmol 2004; 25: 207–213.
Zimmerman TJ, Kooner KS, Ford VJ, Olander KW, Mandlekorn RM, Rawlings EF et al. Trabeculectomy vs. nonpenetrating trabeculectomy: a retrospective study of two procedures in phakic patients with glaucoma. Ophthalmic Surg 1984; 15: 734–740.
Gilmour DF, Manners TD, Devonport H, Varga Z, Solebo AL, Miles J . Viscocanalostomy versus trabeculectomy for primary open angle glaucoma: 4-year prospective randomized clinical trial. Eye (Lond) 2009; 23: 1802–1807.
Mermoud A, Schnyder CC, Sickenberg M, Chiou AG, Hediguer SE, Faggioni R . Comparison of deep sclerectomy with collagen implant and trabeculectomy in open-angle glaucoma. J Cataract Refract Surg 1999; 25: 323–331.
El Sayyad F, Helal M, El Kholify H, Khalil M, El Maghraby A . Nonpenetrating deep sclerectomy versus trabeculectomy in bilateral primary open-angle glaucoma. Ophthalmology 2000; 107: 1671–1674.
Shaarawy T, Mermoud A . Deep sclerectomy in one eye vs deep sclerectomy with collagen implant in the contralateral eye of the same patient: long-term follow-up. Eye 2005; 19: 298–302.
Bissig A, Rivier D, Zaninetti M, Shaarawy T, Mermoud A, Roy S . Ten years follow-up after deep sclerectomy with collagen implant. J Glaucoma 2008; 17: 680–686.
Galassi F, Giambene B . Deep sclerectomy with SkGel implant: 5-year results. J Glaucoma 2008; 17: 52–56.
Anand N, Pilling R . Nd:YAG laser goniopuncture after deep sclerectomy: outcomes. Acta Ophthalmol 2010; 88: 110–115.
Mermoud A, Karlen ME, Schnyder CC, Sickenberg M, Chiou AG, Hediguer SE et al. Nd:Yag goniopuncture after deep sclerectomy with collagen implant. Ophthalmic Surg Lasers 1999; 30: 120–125.
Kozobolis VP, Christodoulakis EV, Tzanakis N, Zacharopoulos I, Pallikaris IG . Primary deep sclerectomy versus primary deep sclerectomy with the use of mitomycin C in primary open-angle glaucoma. J Glaucoma 2002; 11: 287–293.
Guedes RA, Guedes VM, Chaoubah A . Factors associated with non-penetrating deep sclerectomy failure in controlling intraocular pressure. Acta Ophthalmol 2011; 89: 58–61.
Anand N, Kumar A, Gupta A . Primary phakic deep sclerectomy augmented with mitomycin C: long-term outcomes. J Glaucoma 2011; 20: 21–27.
Rebolleda G, Munoz-Negrete FJ . Deep sclerectomy with mitomycin C in failed trabeculectomy. Eye 2007; 21: 23–28.
Choudhary A, Wishart PK . Non-penetrating glaucoma surgery augmented with mitomycin C or 5-fluorouracil in eyes at high risk of failure of filtration surgery: long-term results. Clin Experiment Ophthalmol 2007; 35 (4): 340–347. Ref type: Generic.
Gedde SJ, Schiffman JC, Feuer WJ, Parrish RK, Heuer DK, Brandt JD . The tube versus trabeculectomy study: design and baseline characteristics of study patients. Am J Ophthalmol 2005; 140: 275–287.
Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL . Treatment outcomes in the tube versus trabeculectomy study after one year of follow-up. Am J Ophthalmol 2007; 143: 9–22.
Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC . Surgical complications in the tube versus trabeculectomy study during the first year of follow-up. Am J Ophthalmol 2007; 143: 23–31.
Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL . Three-year follow-up of the tube versus trabeculectomy study. Am J Ophthalmol 2009; 148: 670–684.
Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL . Three-year follow-up of the tube versus trabeculectomy study. Am J Ophthalmol 2009; 148: 670–684.
Jabs DA . Improving the reporting of clinical case series. Am J Ophthalmol 2005; 139: 900–905.
Anand N, Arora S, Clowes M . Mitomycin C augmented glaucoma surgery: evolution of filtering bleb avascularity, transconjunctival oozing, and leaks. Br J Ophthalmol 2006; 90: 175–180.
Marquardt D, Lieb WE, Grehn F . Intensified postoperative care versus conventional follow-up: a retrospective long-term analysis of 177 trabeculectomies. Graefes Arch Clin Exp Ophthalmol 2004; 242: 106–113.
Siriwardena D, Kotecha A, Minassian D, Dart JK, Khaw PT . Anterior chamber flare after trabeculectomy and after phacoemulsification. Br J Ophthalmol 2000; 84: 1056–1057.
Chiou AG, Mermoud A, Jewelewicz DA . Post-operative inflammation following deep sclerectomy with collagen implant versus standard trabeculectomy. Graefes Arch Clin Exp Ophthalmol 1998; 236: 593–596.
Katz LJ, Myers JS, Fudemberg SJ . The perils of glaucoma surgical outcome analysis. Am J Ophthalmol 2009; 148: 634–635.
Caprioli J . The tube versus trabeculectomy study: why its findings may not change clinical practice? Am J Ophthalmol 2011; 151: 742–744.
Anand N, Arora S . Surgical revision of failed filtration surgery with mitomycin C augmentation. J Glaucoma 2007; 16: 456–461.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Anand, N., Wechsler, D. Deep sclerectomy with mitomycin C in eyes with failed glaucoma surgery and pseudophakia. Eye 26, 70–79 (2012). https://doi.org/10.1038/eye.2011.238
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2011.238
Keywords
This article is cited by
-
Deep sclerectomy and trabeculectomy augmented with Mitomycin C: 2-year post-operative outcomes
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Mitomycin
Reactions Weekly (2018)
-
Long-term outcomes of needle revision of failing deep sclerectomy blebs
Graefe's Archive for Clinical and Experimental Ophthalmology (2015)
-
Interval censoring and competing risks when reporting results of glaucoma surgery
Eye (2014)
-
Interval censoring for survival curves when reporting the results of glaucoma surgery
Eye (2013)