Abstract
Purpose
To assess the effect of hydroxypropyl (HP)-Guar added to regular post-phacoemulsification treatment in dry eye signs and symptoms, and its influence on the expression of various inflammatory markers by flow cytometry (FCM) in impression cytology specimens.
Methods
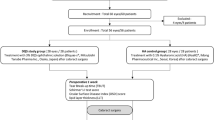
This prospective, interventional, single-centre study included 48 eyes of 48 patients with age-related cataract. After phacoemulsification, patients were randomised to the usual treatment group (UT), with 21 patients who received tobramycin and dexamethasone eye drops (Tobradex, Alcon Cusí, Spain), and the HP-Guar group, with 27 patients who received the UT plus preservative-free artificial tears (Systane UD, Alcon Cusí, Spain). Corneal and conjunctival staining with fluorescein and lissamine green, tear film break-up time (TBUT), Schirmer's I test with anaesthesia (Jones test), tear clearance, and ocular surface disease index (OSDI) were assessed preoperatively and 1 month after surgery. Besides, conjunctival impression cytology was performed in order to investigate inflammatory markers (CD3, CD11b, and HLA-DR) using FCM.
Results
HP-Guar group shows statistical better results compared with the UT group in TBUT (6.4±0.7 vs9±2.5, P=0.0004), OSDI (11.5±8.2 vs3.3±2.5, P=0.0002), ocular symptoms subscale (7.3±6.1 vs1.7±1.8, P=0.0004), vision-related function subscale (2.2±1.8 vs0.4±0.6, P=0.0002), CD3 (2.5±1.4 vs1.1±1.1, P=0.011), and HLA-DR (6.8±4.5 vs1.8±1.7, P=0.0002).
Conclusion
The addition of HP-Guar to regular treatment after cataract surgery reduces ocular surface inflammation and dry eye signs and symptoms.
Similar content being viewed by others
Introduction
Dry eye is a very common condition, one of the most frequently encountered worldwide, with 4.3 million people over 65 years of age experiencing dry eye symptoms in the United States.1 It usually appears with symptoms of irritation, eye redness, foreign body sensation, and ocular fatigue. A new definition of this disease has been developed recently, due to evidence which supports that inflammation has a key role in the development of dry eye.2, 3, 4, 5 Dry eye is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.6 Impaired functional visual acuity has been reported in dry eye patients, with difficulty in reading and driving due to ocular surface irregularity secondary to unstable tear film.7, 8 Thus, dry eye can significantly interfere in patients’ quality of life (QOL).9
Phacoemulsification is the routine technique in cataract surgery nowadays. Tear film changes have been reported after phacoemulsification.10, 11 The incidence of dry eye signs and symptoms has been shown to increase dramatically after cataract surgery. Effective treatment, such as artificial tears or lacrimal plugs, has been recommended in dry eye patients after phacoemulsification.12
Hydroxypropyl (HP)-Guar (Systane UD, Alcon Cusí, Spain) are preservative-free artificial tears with proven efficacy in dry eye patients, improving tear film break-up time (TBUT),13 corneal staining,14 corneal permeability,15 and patients’ symptoms.13
Flow cytometry (FCM) is a widely used technique for investigating ocular surface biology and pathophysiology. Studies performed on impression cytology with FCM techniques confirmed an inflammatory pattern in a large number of patients with dry eye.16, 17, 18, 19
The purpose of this study was to assess the effect of HP-Guar added to conventional post-phacoemulsification treatment in dry eye signs and symptoms, and its influence on the expression of various inflammatory markers by FCM in impression cytology specimens.
Patients and methods
Patients
This prospective, interventional, randomised, single-centre study included 48 eyes of 48 patients over 60 years of age who were consecutively scheduled for age-related cataract surgery. Exclusion criteria included cataract with non-age-related aetiology (traumatic, uveitic, drug-induced, and so on), history of herpetic keratitis or any other disease of the ocular surface, previous eye surgery, contact lens wear, use of any preoperative topical eye drops (including artificial tears, glaucoma meds, steroids, NSAIDs, and antihistamines), presence of punctal plugs or cautery punctal occlusion, pseudoexfoliation, and intraocular pressure above 21 mm Hg. Patients with systemic diseases predisposing them to dry eye were also excluded from the study.
All patients underwent uneventful cataract surgery with phacoemulsification and in-the-bag intraocular lens implantation. No antibiotic topical treatment was used before the surgery. Tropicamide 1% (Tropicamida, Alcon Cusí, Spain) and phenylephrine 10% (Fenilefrina, Alcon Cusí, Spain) were used three times over half an hour to dilate the pupil before the surgery. Oxybuprocaine and tetracaine (Anestésico doble, Alcon Cusí, Spain) were used as surface anaesthesia. No intracameral anaesthesia was used.
Patients were randomly assigned by a computer-generated randomisation list to two treatment groups after cataract surgery. The usual treatment group (UT) included 21 patients who received our usual post-operative treatment with tobramycin and dexamethasone topical eye drops (Tobradex, Alcon Cusí, Spain) four times a day for the first week, tapering the dose for a further 3 weeks; and the HP-Guar group included 27 patients who received the UT plus preservative-free artificial tears (Systane UD) four times a day for 1 month.
The marketed eye drop solution Systane UD was used in this study. Systane UD contains polyethylene glycol 400 0.4% and propylene glycol 0.3% as active demulcents with HP-Guar as a gelling agent. Systane UD also contains some essential ions (calcium, potassium, magnesium, sodium, and zinc). Systane UD was supplied in its original sterile, single-use, single-dose container with no preservatives.
Clinical assessments
Patients underwent a clinical examination preoperatively and 1 month after surgery. At each study visit, the following parameters were assessed: corneal staining with fluorescein, conjunctival staining with lissamine green, TBUT, Schirmer I test with anaesthesia (Jones test), and tear clearance. Staining of the cornea was assessed with a 0.5% fluorescein solution and lissamine green strips using the Oxford Scheme for grading ocular surface staining.20 Schirmer's I test with anaesthesia (Jones test) and tear clearance were assessed simultaneously by our previously described colorimetric technique. For this assessment, one drop each of fluorescein 0.5% and oxybuprocaine 0.4% eye drops was instilled and after 5 min a strip of Schirmer's paper was placed, and another 5 min pause was taken. At the strip reading we obtained: the value of Schirmer's test from the length of the wet mark and the value of tear clearance by comparing the colour of the wet part with the colours of a colorimetric chart.21
Moreover, conjunctival impression cytology was performed in order to investigate inflammatory markers (CD3, CD11b, and human leukocyte antigen-DR (HLA-DR)) using FCM.
All the patients were also asked to complete the OSDI (ocular surface disease index) questionnaire .22 OSDI evaluates the effect of dry eye on QOL items divided into three subscales: ocular symptoms, such as gritty or painful eyes; vision-related function, which measures the limitations in performing everyday tasks, such as reading or using a computer; and response to environmental triggers, such as the wind. It uses a 4-grade scale: never, 0; sometimes, 1; half the time, 2; most of the time, 3; all the time, 4. Total OSDI was expressed as [(sum of the scores of all answered questions) × 100]/[(total no. of answered questions) × 4]. The final score is on a scale of 0–100, where a lower score implies fewer disability. Subscale scores were similarly calculated with select questions from each subscale.
Visual acuity and ophthalmic examinations, including slit lamp biomicroscopy, intraocular pressure, and reporting adverse effects throughout the study, were assessed as safety parameters.
Conjunctival impression cytology
Specimens were collected more than 15 min after the last dye test. After applying one drop of topical anaesthetic (Anestésico doble colirio), one 0.20 μm polyethersulfone filter membrane (Supor Membranes, Gelman Sciences, Ann Arbor, MI, USA) was applied to the superotemporal bulbar conjunctiva without exerting any pressure. Care was taken to collect specimens only in non-exposed regions of the conjunctiva. Membranes were immediately removed after contact. The membranes had to be transferred into tubes containing 2 ml of phosphate-buffered saline (PBS) and 0.05% paraformaldehyde. The tubes were kept at 4°C before and after collection.
Flow cytometry
Membranes were processed for an immunofluorescence procedure. They were first incubated at room temperature for 30 min with mouse monoclonal antibodies directed against cell surface markers. These antibodies were obtained from Invitrogen (Carlsbad, CA, USA): CD11b, mouse anti-human, (APC), CD3, mouse anti-human, (FITC) and HLA-DR (Class II), mouse anti-human (PE-Cy5.5). CD11b binds to CD18 to form the heterodimeric complex known as Mac-1. This complex serves as a receptor for the iC3b component. Mac-1 also serves as an adhesion molecule for intracellular adhesion molecule-1, also known as CD54. Mac-1 is expressed on cells of the myeloid lineage as well as natural killer cells. The CD3 molecule is a complex consisting of at least five glycoprotein chains, each having a molecular weight of 20–25 kDa. The γ-subunit and likely other subunits of this molecule are closely associated with the α- and β-chains of the T-cell receptor (TCR) molecule. The CD3/TCR complex is responsible for the recognition of antigens, which are expressed in association with the major histocompatibility complex (MHC) antigens. The CD3 molecule is present on the majority of resting and activated mature T lymphocytes, and has been used extensively to enumerate these cells in human peripheral blood. The mouse anti-human HLA-DR (Class II) antibody recognises the class II HLA-DR. These antigens are expressed on B cells, activated T cells, dendritic cells, epithelial cells, and macrophages, and are a marker of inflammatory state.
Filter membranes in Eppendorf tubes with PBS were sonicated for 3 min at room temperature for the staining. The tubes were centrifuged at 300 g for 5 min and washed twice. The pellet was resuspended in 100 μl of PBS with 0.1% BSA and antibodies were added (10 μl of each one). Incubation time was 15 min at room temperature. After that, incubation cells were washed twice, once again in PBS (0.1% BSA), and analysed by FCM.
The flow cytometer was a CyAn ADP Analyzer (Beckman Coulter, Hialeah, FL, USA) equipped with three lasers and nine fluorescence detectors. FITC, PE, and PE-Cy5 were excited with a 488 nm solid-state laser and APC with a 633 nm diode laser. At least 10 000 cells were acquired per sample. Data analysis was performed with Summit Software version 4.3 (Beckman Coulter). Results were calculated as percentages of positive cells.
Statistics
The results were collected in an Excel 2000 spreadsheet (Microsoft Corporation, Redmond, WA, USA) and analysed with the statistical program SPSS Statistics for Windows, version 9.0 (SPSS Science, Chicago, IL, USA). Numerical variables are expressed as average and standard deviation, and the categorical data as percentage. Student's t-test was performed to compare the two groups and the paired Student's t-test was used on paired observations. The χ2-test was used for categorical data. A P<0.05 was considered statistically significant.
Written informed consent was obtained from each patient before the baseline measurements. The tenets of the Declaration of Helsinki and Spanish legislation were adhered to and the study protocol was approved by the local Ethics Committee.
Results
Overall, 48 eyes of 48 patients were included in the study. Cataract surgery (phacoemulsification and intraocular lens implant) was performed without any incident in all the patients.
There were no differences between the two groups in terms of demographic characteristics nor were there any differences in the preoperative parameters (Table 1).
Table 2 displays the data from both groups 1 month after the cataract surgery. There were statistically significant differences in favour of the HP-Guar group in the TBUT, overall OSDI, ocular symptoms subscale, vision-related function subscale, CD3, and HLA-DR, and better overall results were obtained in the HP-Guar group.
UT patient data after the surgery showed significant differences for preoperative parameters in triggers OSDI, CD3, and CD11b, which were the only parameters that improved after the surgery in this group (Table 3). In contrast, all the parameters improved after phacoemulsification in the HP-Guar group, with statistically significant differences in the TBUT, overall OSDI, ocular symptoms subscale, vision-related function subscale, HLA-DR, and CD11b (Table 4).
There were no adverse events related to the added treatment.
Discussion
This study demonstrates that adding HP-Guar to regular treatment after cataract surgery by phacoemulsification reduces conjunctival epithelial cell expression of inflammatory markers, while reducing the signs (TBUT) and symptoms (overall OSDI, ocular symptoms, and vision-related function subscales) of dry eye experienced by the majority of operated patients.
Dry eye patients presented an inflammatory response with conjunctival lymphocytic infiltration,23 which explains the increase in the expression of inflammatory markers in these patients.24 Among these markers, we can find the expression of the MHC HLA-DR class II antigens as the most relevant factor. HLA-DR expression is normally restricted to immune cells but could be overexpressed by epithelial cells if immune-driven inflammation occurs. Moreover, HLA-DR has a high sensitivity depending on the degree of inflammatory reactions; it is one of the best evaluation standards of ocular surface inflammation. CD3 is a marker of T cells, cells which are also involved in the inflammation of the ocular surface associated with dry eye. Elevated levels of this marker have been found in dry eye patients.25 Treating dry eyes with artificial tears has proven to reduce the expression of these inflammatory markers,26 which could be a consequence of the increased stability of the tear film upon increasing the TBUT and of the subsequent reduction in hyperosmolarity that the lubricant treatment produces. Our study indicates an increase in HLA-DR and CD3 after cataract surgery, which is insignificant in the case of HLA-DR. This seems to point to the existence of an increased inflammation of the ocular surface in operated patients, similar to the inflammation that appears with dry eye. Both HLA-DR and CD3 markers show a significant difference in favour of the HP-Guar group 1 month after surgery. This indicates a decrease in ocular surface inflammation in those patients who received HP-Guar in addition to regular post-operative treatment. Moreover, these patients displayed increased tear film stability a month after surgery, demonstrating TBUT improvement, the difference in TBUT in the two groups being statistically significant 1 month after surgery. Reduced inflammatory activity and improved tear film quality translates to a better mark on the OSDI questionnaire for the patients in the HP-Guar group; the difference in vision-related function subscales and ocular symptoms is also significant. In other words, patients who received HP-Guar after surgery had less ocular symptoms and were less limited in daily activities, such as reading. The significant improvement in the vision-related function subscale presented by the patients in the HP-Guar group may be due to the improvement in the regularity of the corneal surface, and not to the surgery itself, as both groups had similar post-operative visual acuity.
Sometimes after cataract surgery, patients complaint about visual fluctuation. This may be caused by dry eye until proven other etiologies such as post-operative corneal oedema, residual astigmatism or refractive error, and cystoid macular oedema. We have already discussed that visual quality begins with a healthy ocular surface and the importance that a stable tear film has for vision, as it is the most important optical interface of the eye.27 Treatment with artificial tears has been reported to increase the regularity of the corneal surface,28 which translates to improved vision.29 In accordance with these reports, in our study, HP-Guar-treated patients had less visual disturbances after phacoemulsification, thanks to lubricant treatment. This is of utmost importance when dealing with multifocal intraocular lens implants.
There are various factors that could contribute to the appearance of dry eye after cataract surgery. Inflammation of the ocular surface can occur after surgery and drugs, the latter producing toxic changes in the cornea and conjunctiva due to the existence of preservatives, particularly benzalkonium chloride.12 A corneal incision, notwithstanding its small size, can cause certain corneal irregularities, favouring tear film ruptures. Moreover, an alteration in central corneal sensitivity has been found in patients who have undergone cataract surgery,30 secondary to the corneal nerve section, which may potentially disrupt the neural loop, reducing tear secretion by the lacrimal gland.31
The increase in TBUT and the improvement of the OSDI and its subscales after treatment with HP-Guar may be due to its peculiar mechanism of action. Upon binding Systane content, stored at a pH of 7.0, with tears, which have a pH between 7.5 and 7.8, borate ions meet with the HP-Guar and form a matrix, which increases the retention of compound's lubricant (PEG 400 and PG), resulting in an increase in the stability of the tear film on the ocular surface, and thus in decreased corneal desiccation32 and permeability.15
In both groups of patients, a significant reduction of the CD11b marker was found. The role of this marker in ocular inflammation is still unknown, even when the existence of this inflammatory marker had already been reported in the cornea of a dry eye animal model, which was the result of leukocytic infiltration and activation.33 The decrease we discovered in both groups could be explained by the corticosteroid treatment patients received in the post-operative period.
There is only one study that determines the effect of an additional treatment after cataract surgery on dry eye signs and symptoms; however, it does not involve artificial tears, but rather cyclosporin A 0.05% twice a day 1 month preoperatively and 1 month post-operatively, resulting in an improvement in symptoms (in a non-validated questionnaire) but not in TBUT, which did not improve in the group receiving CyA.34 A recent study has shown similar results adding CyA after trabeculectomy, improving OSDI index with neither TBUT nor Schirmer test values improvement.35
Nowadays, in the phacorefractive age, cataract surgeons must be interested in further improving the outcomes of cataract surgery by decreasing symptoms such as burning or foreign body sensation and by improving the quality of vision after surgery, which is associated to ocular dryness. By aggressively treating the ocular surface after surgery (particularly but not only in patients with previous problems36), we provide better patient comfort and visual acuity. We must inform patients about the possibility of ocular dryness symptoms and visual fluctuation after surgery, in order to avoid patient complaints about cataract surgery. Recently, an ‘ocular surface stress test’ has been reported to identify high-risk patients for developing dry eye signs and symptoms after phacoemulsification.37 In spite of this, and because of the high prevalence of dry eye after phacoemulsification and the positive results of adding HP-Guar treatment, we recommend lubricant treatment at least during the first month after phacoemulsification in all the patients undergoing cataract surgery.
To our knowledge, this study is the first to study the inflammatory markers of the ocular surface after cataract surgery and the difference in these markers after adding lubricant treatment. Increased inflammation of the ocular surface in patients after cataract surgery maintains the high occurrence of dry eye in said patients. Likewise, the improvement of said inflammatory markers appears in the group treated with HP-Guar, together with the increased stability of the tear film reflected in the TBUT, and the decrease in symptoms that patients in this group experience, objectified by the OSDI questionnaire, support the need to add a lubricant treatment during the post-operative cataract surgery (by phacoemulsification) period. By reducing these symptoms we increase patient satisfaction and his/her QOL, a factor that can be compromised by dry eye. The mechanism of action of HP-Guar, together with the absence of preservatives, makes it a suitable treatment in these cases.
To conclude, adding HP-Guar to regular treatment after cataract surgery reduces ocular surface inflammation and dry eye signs and symptoms.

References
Schein OD, Munoz B, Tielsch JM, Bandeen-Roche K, West S . Prevalence of dry eye among elderly. Am J Ophthalmol 1997; 124: 723–728.
Baudouin C, Haouat N, Brignole F, Bayle J, Gastaud P . Immunopathological findings in conjunctival cells using immunofluorescence staining of impression cytology specimens. Br J Ophthalmol 1992; 76: 545–549.
Mircheff AK, Wood RL, Gierow JP . Traffic of major histocompatibility complex class II molecules in rabbit lacrimal gland acinar cells. Invest Ophthalmol Vis Sci 1994; 35: 3943–3953.
Tsubota K, Fukagawa K, Fujihara T, Shimmura S, Saito I, Saito K et al. Regulation of human leukocyte antigen expression in human conjunctival epithelium. Invest Ophthalmol Vis Sci 1999; 40: 28–34.
Baudouin C, Brignole F, Becquet F, Pisella PJ, Goguel A . Flow cytometry in impression cytology specimens: a new method for evaluation of conjunctival inflammation. Invest Ophthalmol Vis Sci 1997; 8: 1458–1464.
The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007; 5: 75–92.
Goto E, Yagi Y, Matsumoto Y, Tsubota K . Impaired functional visual acuity of dry eye patients. Am J Ophthalmol 2002; 133: 181–186.
Liu Z, Pflugfelder S . Corneal surface regularity and the effect of artificial tears in aqueous tear deficiency. Ophthalmology 1999; 106: 939–943.
Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W . Utility assessment among patients with dry eye disease. Ophthalmology 2003; 110: 1412–1419.
Liu Z, Luo L, Zhang Z, Cheng B, Zheng D, Chen W et al. Tear film changes after phacoemulsification. Zhonghua Yan Ke Za Zhi 2002; 38: 2747.
Liu X, Yang-Shun GU, Ye-Sheng XU . Changes of tear film and tear secretion after phacoemulsification in diabetic patients. J Zhejiang Univ Sci B 2008; 9: 324–328.
Li XM, Hu L, Hu J, Wang W . Investigation of dry eye disease and analysis of the phatogenic factors in patients after cataract surgery. Cornea 2007; 26: S16–S20.
Ousler GW, Michaelson C, Christensen MT . An evaluation of tear film breakup time extension and ocular protection index scores among three marketed lubricant eye drops. Cornea 2007; 26: 949–952.
Christensen MT . Corneal staining reductions observed after treatment with Systane lubricant eye drops. Adv Ther 2008; 25: 1191–1199.
Cervan-Lopez I, Saenz-Frances-San-Baldomero F, Benitez-del-Castillo JM, Garcia-Sanchez J . Reduction of corneal permeability in patients treated with HP-Guar: A fluorophotometric study. Arch Soc Esp Oftalmol 2006; 81: 327–332.
Brignole-Baudouin F, Ott AC, Warnet JM, Baudouin C . Flow cytometry in conjunctival impression cytology: a new tool for exploring ocular surface pathologies. Exp Eye Res 2004; 78: 473–481.
Brignole F, de Saint-Jean M, Goldschild M, Becqet F, Goguel A, Baudouin C . Expression of Fas-Fas ligand antigens and apoptotic marker APO2-7 by the human conjunctival epithelium. Positive correlation with class II HLA DR expression in inflammatory ocular surface disorders. Exp Eye Res 1998; 67: 687–697.
Pisella PJ, Brignole F, Debbasch C, Lozat P, Garcher C, Bara J et al. Flow cytometric analysis of conjunctival epithelium in occular rosacea and keratoconjunctivitis sicca. Ophthalmology 2000; 107: 1841–1849.
Brignole F, Pisella PJ, De Saint Jean M, Goldschild M, Goguel A, Baudouin C . Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporin A. Invest Ophthalmol Vis Sci 2001; 112: 1446–1454.
Bron AJ, Evans VE, Smith JA . Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003; 22: 640–650.
Vico E, Benítez del Castillo JM, Jiménez RA, Fernández C, García Sánchez J . Tear function index validation for dry eye diagnosis. Arch Soc Esp Oftalmol 2004; 79: 265–271.
Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL . Reliability and validity of ocular surface disease index. Arch Ophthalmol 2001; 119: 456.
Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC . The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea 1998; 17: 584–589.
Brignole F, Pisella PJ, Goldschild M, De Saint Jean M, Goguel A, Baudouin C . Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci 2000; 41: 1356–1363.
Stern ME, Gao J, Schwalb TA, Ngo M, Tieu DD, Chan CC et al. Conjunctival T-cell subpopulations in Sjögren's and non-Sjögren's patients with dry eye. Invest Ophthalmol Vis Sci 2002; 43: 2609–2614.
Kunert KS, Tisdale AS, Stern ME, Smith JA, Gipson IK . Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol 2000; 118: 1489–1496.
Montés-Micó R . Role of the tear film in the optical quality of the human eye. J Cataract Refract Surg 2007; 33: 1631–1635.
Liu Z, Pflugfelder S . Corneal surface regularity and the effect of artificial tears in aqueous tear deficiency. Ophthalmology 1999; 106: 939–943.
Nilforoushan MR, Latkany RA, Speaker MG . Effect of artificial tears on visual acuity. Am J Ophthalmol 2005; 140: 830–835.
Khanal S, Tomlison A, Esakowitz L, Bhatt P, Jones D, Nabili S et al. Changes in corneal sensitivity and tear physiology after phacoemulsification. Ophthal Physiol Opt 2008; 28: 127–134.
Kohlhaas M . Corneal sensation after cataract and refractive surgery. J Cataract Refract Surg 1998; 24: 1399–1409.
Ubels JL, Clousing DP, Van Haitsma TA, Hong BS, Stauffer P, Asgharian B et al. Pre-clinical investigation of the efficacy of an artificial tear solution containing hydroxypropyl-guar as a gelling agent. Curr Eye Res 2004; 28: 437–444.
Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR . Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol 2008; 126: 219–225.
Roberts CW, Elie ER . Dry eye symptoms following cataract surgery. Insight 2007; 32: 14–21.
Fakhraie G, Lopes JF, Spaeth GL, Almodin J, Ichhpujani P, Moster MR . Effects of postoperative cyclosporine ophthalmic emulsion 0.05% (Restasis) following glaucoma surgery. Clin Experiment Ophthalmol 2009; 37: 842–848.
Ram J, Gupta A, Brar GS, Kaushik S, Gupta A . Outcomes of phacoemulsification in patients with dry eye. J Cataract Refract Surg 2002; 28: 1386–1389.
Hardten DR . Dry eye disease in patients after cataract surgery. Cornea 2008; 27: 855.
Acknowledgements
This study was supported by GR-UCM research group 920415 (GR58/08). Treatment was provided by Alcon.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sánchez, M., Arriola-Villalobos, P., Torralbo-Jiménez, P. et al. The effect of preservative-free HP-Guar on dry eye after phacoemulsification: a flow cytometric study. Eye 24, 1331–1337 (2010). https://doi.org/10.1038/eye.2010.24
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.24
Keywords
This article is cited by
-
New Therapeutic Strategy and Innovative Lubricating Ophthalmic Solution in Minimizing Dry Eye Disease Associated with Cataract Surgery: A Randomized, Prospective Study
Advances in Therapy (2020)
-
Effects of cataract surgery on symptoms and findings of dry eye in subjects with and without preexisting dry eye
Japanese Journal of Ophthalmology (2020)
-
Changes in ocular surface status after phacoemulsification in patients with senile cataract
International Ophthalmology (2019)
-
Hydroxypropyl methylcellulose 2% for dry eye prevention during phacoemulsification in senile and diabetic patients
International Ophthalmology (2018)
-
Impact of polyethylene glycol 400/propylene glycol/hydroxypropyl-guar and 0.1% sodium hyaluronate on postoperative discomfort following cataract extraction surgery: a comparative study
Eye and Vision (2017)