Abstract
In this study, we observed that lysophosphatidylglycerol (LPG) completely inhibited a formyl peptide receptor like-1 (FPRL1) agonist (MMK-1)-stimulated chemotactic migration in human phagocytes, such as neutrophils and monocytes. LPG also dramatically inhibited IL-1β production by another FPRL1 agonist serum amyloid A (SAA) in human phagocytes. However, LPG itself induced intracellular calcium increase and superoxide anion production in human phagocytes. Keeping in mind that phagocytes migration and IL-1β production by FPRL1 are important for the induction of inflammatory response, our data suggest that LPG can be regarded as a useful material for the modulation of inflammatory response induced by FPRL1 activation.
Similar content being viewed by others
Introduction
Formyl peptide receptor like-1 (FPRL1) is one of the classic chemoattractant receptors and is made up of a seven trans-membrane-spanning G protein-coupled receptor (Le et al., 2001, 2002). FPRL1 is highly expressed on phagocytic cells such as neutrophils, monocytes, and dendritic cells (Le et al., 2001, 2002). In terms of functional activity of FPRL1, it has been found to mediate the chemotactic migration of phagocytes in a pertussis toxin (PTX)-sensitive manner, thus indicating the receptor couple to the Gi subfamily of G proteins (Le et al., 2001, 2002). FPRL1 also plays an important role in immunological function, including host defenses against pathogen infection (Le et al., 2001, 2002). The ligands for FPRL1 include MMK-1, LL-37, and serum amyloid A (SAA) (Klein et al., 1998; Su et al., 1999; Yang et al., 2000; Le et al., 2001). Since FPRL1 is involved in the recruitment of several leukocytic cells into infected or inflammatory sites, it has been important issue to identify a molecule that inhibits FPRL1-mediated leukocyte trafficking.
Lysophospholipids including lysophosphatidic acid (LPA) act as lipid ligands that stimulate several cellular responses (Graler and Goetzl, 2002; Steiner et al., 2002). In case of LPA, it has been reported to induce cellular proliferation, cellular migration and invasion in several cell types including ovarian cancer cells (Xie et al., 2002; Ren et al., 2006). Among these lysophospholipids, lysophosphatidylglycerol (LPG) has been reported to prevent binding of LPA to a putative LPA receptor on cell-surface of mouse NIE-115 neuroblastoma cells (van der Bend et al., 1992). A past report showed that high concentration of LPG (60 µM) blocked intracellular calcium increase induced by LPA in HEY ovarian cancer cells (Xu et al., 1995). We also reported that LPG stimulates several signaling molecules, including intracellular calcium increase, ERK, and Akt, in human ovarian cancer cells (Park et al., 2007). LPG also stimulates the chemotactic migration and tube formation in human umbilical vein endothelial cells (Lee et al., 2007). In both studies, we suggested that LPG utilizes G-protein coupled receptors other than the known LPA receptors, by showing no response to LPG in each LPA receptor expressing HepG2 cells and no blockage of LPG response with the selective LPA receptor antagonist, Ki16425. However, roles of LPG in the modulation of immunological responses induced by certain chemoattractants have not been studied.
In this study we investigated the effect of LPG on the recruitment of leukocytes and an inflammatory cytokine production from human phagocytes by FPRL1 activation. We found that LPG could inhibit FPRL1-induced cellular responses from human phagocytes. We anticipate that this lipid has the potential as a useful material for the modulation of FPRL1-mediated (patho) physiological responses.
Results
LPG inhibits MMK-1-mediated chemotactic migration in human neutrophils
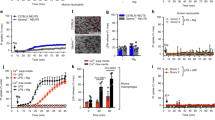
Neutrophils are key players for the immune responses against invading pathogens. Chemotactic migration is representative of the function of neutrophils. Neutrophil chemotaxis was dramatically induced by MMK-1, a well-known agonist for FPRL1, showing a concentration-dependency (Figure 1A). Maximal activity was observed at 1 µM of MMK-1 (Figure 1A). We also examined the effect of LPG on neutrophil chemotactic migration. When human neutrophils were stimulated with several concentrations of LPG, it did not affect chemotactic migration of human neutrophils (Figure 1B). Next, we checked whether LPG affect chemotactic migration of neutrophils by MMK-1. The preincubation of human neutrophils with several concentrations of LPG prior to the addition of MMK-1, resulted in the inhibition of the MMK-1- induced neutrophil chemotaxis (Figure 1C). 10-20 µM of LPG almost completely inhibited MMK-1- induced neutrophil chemotaxis (Figure 1C).
Effect of LPG on MMK-1-induced chemotactic migration in human neutrophils. Isolated human neutrophils (1 × 106 cells/ml of serum free RPMI 1640 medium) were added to the upper wells of a 96-well chemotaxis chamber. Various concentrations of MMK-1 or LPG were used for the chemotaxis (A, B). Chemotaxis assay was performed using MMK-1 in the presence of several concentrations (0, 1, 5, 10, and 20 µM) of LPG in the upper chamber (C). The migration across a 3 µm pore sized polycarbonate membrane was assessed after incubating at 37℃ for 1.5 h. The number of migrated cells was determined by counting the number of cells in 5 high power fields (400×). The data are expressed as the means ± SE from three independent experiments performed in duplicate (A-C). *P < 0.05 compared to the values obtained from the control (0 µM). #Significantly different from the control (MMK-1 only treated) (P < 0.05).
LPG inhibits MMK-1-induced chemotactic migration in human monocytes
In addition to neutrophils, monocytes also play an important role in immune responses. The activation of FPRL1 by its specific agonist (MMK-1) induced monocyte chemotaxis. MMK-1-induced monocyte chemotaxis was concentration-dependent, showing maximal activity at 1 µM (Figure 2A). We also tested the effect of LPG on the migration of human monocytes. LPG (1-20 µM) alone failed to stimulate monocytes chemotaxis (Figure 2B). Then, we checked the effect of LPG on the MMK-1-induced monocyte chemotaxis. When human monocytes were preincubated with several concentrations of LPG, MMK-1-induced monocyte chemotaxis was dramatically inhibited showing concentration-dependency (Figure 2C). 10-20 µM of LPG totally inhibited MMK-1-induced monocyte chemotaxis (Figure 2C).
Effect of LPG on MMK-1-induced chemotactic migration in human monocytes. Isolated human monocytes (1 × 106 cells/ml of serum free RPMI 1640 medium) were added to the upper wells of a 96-well chemotaxis chamber. Various concentrations of MMK-1 or LPG were used for the chemotaxis (A, B). Chemotaxis assay was performed using MMK-1 in the presence of several concentrations (0, 1, 5, 10, and 20 µM) of LPG in the upper chamber (C). The migration across a 5 µm pore sized polycarbonate membrane was assessed after incubating at 37℃ for 2 h. The number of migrated cells was determined by counting the number of cells in 5 high power fields (400×). The data are expressed as the means ± SE from three independent experiments performed in duplicate (A-C). *P < 0.05 compared to the values obtained from the control (0 µM). #Significantly different from the control (MMK-1 only treated) (P < 0.05).
LPG inhibits SAA-induced IL-1β production in human monocytes and neutrophils
In the initiation of immune responses and inflammatory responses the production of various cytokines are very important (Lin and Karin, 2007). Previously we demonstrated that the activation of FPRL1 elicits the production of some cytokines (Lee et al., 2006, 2008). Here we also found that stimulation of human monocytes with SAA (an FPRL1 agonist) induced one inflammatory cytokine (IL-1β) in a concentration-dependent manner from human monocytes (Figure 3A). SAA-induced IL-1β production was maximal at 1-2 µM (Figure 3A). Another FPRL1 (MMK-1) failed to stimulate IL-1β production from human monocytes (Data not shown). We also examined the role of LPG on IL-1β production from human monocytes. LPG alone did not affect on IL-1β production from human monocytes (Figure 3B). Then, we investigated the effect of LPG on the FPRL1-mediated cytokine production in human monocytes using SAA (an FPRL1 agonist). Preincubation of human monocytes with several concentrations of LPG prior to addition of SAA dramatically inhibited IL-1β production (Figure 3C). 20 µM of LPG inhibited SAA-induced IL-1β production by around 63% from human monocytes (Figure 3C).
The effects of LPG on SAA-induced IL-1β production from human phagocytes. Human monocytes were stimulated with several concentrations of SAA (A) or LPG (B) for 24 h. The cells were treated with vehicle alone or SAA (100 nM) for 24 h in the presence or absence of several concentrations (0, 1, 10, and 20 µM) (C). Secreted IL-1β levels were determined by ELISA (A-C). The data represent the mean ± SE of three independent experiments performed in duplicate (A-C). Human neutrophils were stimulated with SAA (2 µM), LPG (20 µM), or LPG (20 µM) + SAA (2 µM) for 24 h (D). *P < 0.05 compared to the values obtained from the control (0 µM). #Significantly different from the control (SAA only treated) (P < 0.05).
We also examined the effect of LPG on IL-1β production from human neutrophils. LPG (upto 20 µM) alone did not stimulate IL-1β production from human neutrophils (Figure 3D). Preincubation of human neutrophils with 20 µM of LPG inhibited SAA-stimulated IL-1β production (Figure 3D).
LPG stimulates calcium influx and superoxide anion production from human neutrophils and monocytes
It has been demonstrated that the activation of cell surface chemoattractant receptors, via specific agonists, cause diverse intracellular signals, including [Ca2+]i increases (Hu et al., 2001). We tested the effect of LPG upon [Ca2+]i in neutrophils. As shown in Figure 4A, the stimulation of neutrophils, 20 µM of LPG, caused a [Ca2+]i increase. A well-known FPRL1 agonist (MMK-1) also dramatically increased calcium signaling in human neutrophils (Figure 4A). We then examined the effect of LPG on the induction of calcium signaling by FPRL1 agonist (MMK-1). As shown in Figure 4A, the preincubation of human neutrophils with 20 µM LPG completely did not affect the MMK-1-induced calcium increases.
The effects of LPG on calcium influx and superoxide anion production from human phagocytes. Fura-2 loaded human neutrophils (A) or monocytes (C) were stimulated with 20 µM of LPG or 1 µM of MMK-1 at the indicated points. The changes in 340 nm / 380 nm were also monitored. The results are representative of three independent experiments. Isolated human neutrophils (B) or monocytes (D) (2 × 106 cells/ml/assay) were preincubated for 1 min at 37℃ with 50 µM of cytochrome c before being stimulated with MMK-1 (1 µM), LPG (20 µM), or MMK-1 (1 µM) + LPG (20 µM) for several lengths of time. Cytochrome c reduction was monitored as a change in absorption at 550 nm at 1 min intervals over 5 min. The data are representative of three independent experiments performed in duplicate (B, D).
Since superoxide is the most important armory on the primary defense line of neutrophils against invading pathogens (Dahlgren and Karlsson, 1999), we examined the role of LPG on the superoxide anion production from human neutrophils. LPG alone stimulates superoxide anion production from human neutrophils. LPG-induced superoxide anion production was comparable with MMK-1-induced one in human neutrophils (Figure 4B). When human neutrophils were stimulated with LPG+MMK-1, much more amount of superoxide anion production was produced from human neutrophils (Figure 4B).
We also tested the effect of LPG upon [Ca2+]i in monocytes. LPG caused a [Ca2+]i increase in human monocytes. MMK-1 also dramatically increased calcium signaling in human monocytes. MMK-1-stimulated [Ca2+]i increase was also observed from LPG-pretreated human monocytes (Figure 4C). Stimulation of human monocytes with LPG elicited superoxide anion production, which was comparable with MMK-1-induced one in the cells. Stimulation of human monocytes with LPG+MMK-1 generated much more amount of superoxide anion production (Figure 4D).
Discussion
Since phagocytes recruitment into infected or inflammatory site is crucial for the induction of innate immune response, and MMK-1 and its specific receptor (FPRL1) have been reported to play a key role in the regulation of phagocyte recruiting, it has been important issue to identify certain molecules that modulate MMK-1 and FPRL1-mediated cellular response. Until now, various kinds of agonists and a few antagonists have been reported for FPRL1 (Le et al., 2001, 2002; Bae et al., 2004). Besides from direct development of FPRL1 ligands, modulation of FPRL1 is possible via identifying a molecule that indirectly regulates FPRL1 activity after binding to other target molecules. Here we demonstrated that LPG inhibited MMK-1-stimulated chemotactic migration in human neutrophils and monocytes.
SAA is a well-known inflammatory molecule (Uhlar and Whitehead, 1999). Past studies have reported SAA binds to FPRL1 and induce proinflammatory cytokine and chemokine production in several cell types including monocytes (Su et al., 1999; Jijon et al., 2005; Lee et al., 2006, 2008). In this study, we also found that SAA stimulates an important inflammatory cytokine, IL-1β (Figure 3A and 3D). We also demonstrated that LPG dramatically inhibited SAA-stimulated IL-1β production from human monocytes and neutrophils. It suggests that LPG can act as a negative modulator for the SAA-induced inflammatory signaling by blocking the induction of IL-1β from human monocytes and neutrophils.
Here, we observed that LPG itself induced calcium influx in human neutrophils and monocytes (Figure 4A and 4C). Moreover LPG did not affect MMK-1-mediated calcium signaling (Figure 4A and 4C). Since FPRL1-induced calcium signaling is mediated by the activation of PLC activation, it suggests that LPG did not block PLC activity induced by FPRL1. However, here we demonstrated that LPG inhibited chemotactic migration of neutrophils induced by MMK-1. From these results we can rule out the possibility that LPG acts directly on FPRL1. It will be reasonable to assume that LPG can bind to unknown target receptor (s), which induce the inhibition of FPRL1-induced chemotactic migration in human neutrophils. It will be interesting to reveal the molecular target for LPG on human neutrophils and monocytes.
Reactive oxygen species including superoxide anion are generated by phagocytic cell upon activation by invading microorganisms or inflammatory debris (Quinn and Gauss, 2004). Because phagocytic cells such as neutrophils play a critical role in innate immune response (especially in the earliest steps in the host defense against invading microorganisms) by generating these reactive oxygen species, it has been important issue to identify certain molecules that regulate superoxide anion production. In this study we demonstrated that LPG alone stimulates superoxide anion production from human neutrophils and monocytes (Figure 4B and 4D). LPG also dramatically enhanced MMK-1-stimulated superoxide anion production from human neutrophils and monocytes. It suggests that LPG can act as a positive modulator for the MMK-1-induced immune activation in terms of superoxide anion production. For the proper activation of superoxide anion from human neutrophils, several signaling molecules required; they include calcium increase, PLA2 and PLD activation (Dana et al., 1998). In this study we demonstrated that LPG increases intracellular calcium increase, and pretreatment of LPG prior to MMK-1 stimulation, causes dramatic increase of intracellular calcium concentration (Figure 4A). The results are well correlated with previous reports that calcium is essential for the production of superoxide anion from human neutrophils.
In conclusion, LPG can be regarded as a regulator for FPRL1-mediated cellular responses. LPG selectively inhibits the chemotactic migration and IL-1β production from human neutrophils and monocytes. With this in mind, we can consider the putative anti-inflammatory role of LPG, through its selective modulation of FPRL1 activity in human neutrophils and monocytes. LPG also can be developed as a useful molecule for the studying of FPRL1-mediated signaling and as a candidate for the treatment of several diseases involving FPRL1.
Methods
Materials
1-acyl-2-hydroxy-sn-glycero-3-phospho-glycerol (LPG) was purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). MMK-1 (LESIFRSLLFRVM) (Klein et al., 1998) was synthesized from Anygen Co., Ltd. (Gwang-ju, Korea). The purity grade of all the synthetic peptides was > 99%. Recombinant human SAA (endotoxin level < 0.1 ng/µg) was purchased from Peprotech (Rocky Hill, NJ). FBS and RPMI 1640 medium were purchased from Invitrogen Corp. (Carlsbad, CA). The peripheral blood mononuclear cell separation medium (Histopaque-1077) was obtained from Sigma (St. Louis, MO), whereas fura-2 pentaacetoxymethylester (fura-2/AM) was obtained from Molecular Probes (Eugene, OR).
Isolation of human neutrophils and monocytes
Peripheral blood was collected from young healthy donors. Peripheral blood mononuclear cells were separated on a Histopaque-1077 gradient. After two washings with PBS without Ca2+ and Mg2+, the peripheral blood mononuclear cells were suspended in 10% FBS containing RPMI 1640 medium and incubated for 60 min at 37℃ to let the monocytes attach to the culture dish. The cells were washed 5 times with warmed RPMI 1640 medium to washout lymphocytes, and then the attached monocytes were collected as described previously (Bae et al., 2001). Human neutrophils were isolated according to the standard procedures of dextran sedimentation, hypotonic lysis of erythrocytes, and a lymphocyte separation medium gradient as described previously (Bae et al., 2001). The isolated human leukocytes were then used promptly.
Chemotaxis assay
Chemotaxis assays were performed using multiwell chambers (Neuroprobe Inc. Gaithersburg, MD), as previously described (Bae et al., 2001; Kim et al., 2007). Briefly, prepared human neutrophils were suspended in RPMI 1640 medium at 1 × 106 cells/ml, and 25 µl of this suspension was placed into the upper well of a chamber, separated by a 3 µm (5 µm for monocytes) polyhydrocarbon filter, from the peptide containing lower well. Following incubation at 37℃ for 1.5 h (2 h for monocytes), the filter was removed, fixed in methanol, and stained with hematoxylin. The cells that migrated across the filter were counted using light microscopy. The migrated cells were stained with hematoxylin and counted in five randomly chosen high power fields (400 ×) (Bae et al., 2001; Kim et al., 2007).
Cytokine assay
Cytokine measurement was performed as previously described (Jo et al., 2004). The monocytes or neutrophils (3 × 106 cells/0.3 ml) were placed in RPMI 1640 medium containing 5% FBS in 24-well plates and kept in a 5% CO2 incubator at 37℃. After stimulation, the cell-free supernatants were collected, centrifuged, and measured for IL-1β by the enzyme-linked immunosorbent assay (BD Biosciences Pharmingen, San Diego, CA) as per the vendor's instructions.
Measurement of intracellular calcium concentration
Intracellular calcium concentration ([Ca2+]i) was determined by Grynkiewicz's method using fura-2/AM (Bae et al., 2003). Briefly, the prepared cells were incubated with a 3 µM fura-2/AM at 37℃ for 50 min in a fresh serum-free RPMI 1640 medium with continuous stirring. Aliquots of 2 × 106 cells were allocated for each assay into Locke's solution (154 mM NaCl, 5.6 mM KCl, 1.2 mM MgCl2, 5 mM HEPES, pH 7.3, 10 mM glucose, 2.2 mM CaCl2, and 0.2 mM EGTA). Fluorescence was measured at 500 nm and at the excitation wavelengths of 340 nm and 380 nm.
Measurement of superoxide anion generation
Superoxide anion generation was determined by measuring cytochrome c reduction using a microtiter 96-well plate ELISA reader (Bio-Tek instruments, EL312e, Winooski, VT) as previously described (Bae et al., 2003). Human neutrophils or monocytes (2 × 106 cells in RPMI 1640 medium) were preincubated with 50 µM of cytochrome c at 37℃ for 1 min and then incubated with each peptide. Superoxide generation was determined by measuring light absorption changes at 550 nm over 5 min at 1 min intervals.
Data analysis
The results are expressed as the means ± SE. The student's t-test was used to compare individual treatments with their respective control values. Statistical significance was set at P < 0.05.
Abbreviations
- [Ca2+]i:
-
intracellular calcium concentration
- FPRL1:
-
formyl peptide receptor like-1
- fura-2/AM:
-
fura-2 pentaacetoxymethylester
- LPA:
-
lysophosphatidic acid
- LPG:
-
lysophosphatidylglycerol
- SAA:
-
serum amyloid A
References
Bae YS, Bae H, Kim Y, Lee TG, Suh PG, Ryu SH . Identification of novel chemoattractant peptides for human leukocytes . Blood 2001 ; 97 : 2854 - 2862
Bae YS, Yi HJ, Lee HY, Jo EJ, Kim JI, Lee TG, Ye RD, Kwak JY, Ryu SH . Differential activation of formyl peptide receptor-like 1 by peptide ligands . J Immunol 2003 ; 171 : 6807 - 6813
Bae YS, Lee HY, Jo EJ, Kim JI, Kang HK, Ye RD, Kwak HY, Ryu SH . Identification of peptides that antagonize formyl peptide receptor-like 1-mediated signaling . J Immunol 2004 ; 173 : 607 - 614
Dahlgren C, Karlsson A . Respiratory burst in human neutrophils . J Immunol Methods 1999 ; 232 : 3 - 14
Dana R, Leto TL, Malech HL, Levy R . Essential requirement of cytosolic phospholipase A2 for activation of the phagocyte NADPH oxidase . J Biol Chem 1998 ; 273 : 441 - 445
Graler MH, Goetzl EJ . Lysophospholipids and their G protein-coupled receptors in inflammation and immunity . Biochim Biophys Acta 2002 ; 1582 : 168 - 174
Hu JY, Le Y, Gong W, Dunlop NM, Gao JL, Murphy PM, Wang JM . Synthetic peptide MMK-1 is a highly specific chemotactic agonist for leukocyte FPRL1 . J Leukoc Biol 2001 ; 70 : 155 - 161
Jijon HB, Madsen KL, Walker JW, Allard B, Jobin C . Serum amyloid A activates NF-kappaB and proinflammatory gene expression in human and murine intestinal epithelial cells . Eur J Immunol 2005 ; 35 : 718 - 726
Jo EJ, Lee HY, Lee YN, Kim JI, Kang HK, Park DW, Baek SH, Kwak JY, Bae YS . Group IB secretory phospholipase A2 stimulates CXC chemokine ligand 8 production via ERK and NF-kappa B in human neutrophils . J Immunol 2004 ; 173 : 6433 - 6439
Kim MK, Park KS, Lee H, Kim YD, Yun J, Bae YS . Phytosphingosine-1-phosphate stimulates chemotactic migration of L2071 mouse fibroblasts via pertussis toxin-sensitive G-proteins . Exp Mol Med 2007 ; 39 : 185 - 194
Klein C, Paul JI, Sauve K, Schmidt MM, Arcangeli L, Ransom J, Trueheart J, Manfredi JP, Broach JR, Murphy AJ . Identification of surrogate agonists for the human FPRL-1 receptor by autocrine selection in yeast . Nat Biotechnol 1998 ; 16 : 1334 - 1337
Lee HY, Kim MK, Park KS, Shin EH, Jo SH, Kim SD, Jo EJ, Lee YN, Lee C, Baek SH, Bae YS . Serum amyloid A induces contrary immune responses via formyl peptide receptor-like 1 in human monocytes . Mol Pharmacol 2006 ; 70 : 241 - 248
Lee HY, Kim SD, Shim JW, Lee SY, Lee H, Cho KH, Yun J, Bae YS . Serum amyloid A induces CCL2 production via formyl peptide receptor-like 1-mediated signaling in human monocytes . J Immunol 2008 ; 181 : 4332 - 4339
Lee SY, Lee HY, Kim SD, Shim JW, Bae YS . Lysophosphatidylglycerol stimulates chemotactic migration and tube formation in human umbilical vein endothelial cells . Biochem Biophys Res Commun 2007 ; 363 : 490 - 494
Le Y, Oppenheim JJ, Wang JM . Pleiotropic roles of formyl peptide receptors . Cytokine Growth Factor Rev 2001 ; 12 : 91 - 105
Le Y, Murphy PM, Wang JM . Formyl-peptide receptors revisited . Trends Immunol 2002 ; 23 : 541 - 548
Lin WW, Karin M . A cytokine-mediated link between innate immunity, inflammation, and cancer . J Clin Invest 2007 ; 117 : 1175 - 1183
Park KS, Kim MK, Im DS, Bae YS . Effect of lysophosphatidylglycerol on several signaling molecules in OVCAR-3 human ovarian cancer cells: involvement of pertussis toxin-sensitive G-protein coupled receptor . Biochem Pharmacol 2007 ; 73 : 675 - 681
Quinn MT, Gauss KA . Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases . J Leukoc Biol 2004 ; 76 : 760 - 781
Ren J, Xiao YJ, Singh LS, Zhao X, Zhao Z, Feng L, Rose TM, Prestwich GD, Xu Y . Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells . Cancer Res 2006 ; 66 : 3006 - 3014
Steiner MR, Urso JR, Klein J, Steiner SM . Multiple astrocyte responses to lysophosphatidic acids . Biochim Biophys Acta 2002 ; 1582 : 154 - 160
Su SB, Gong W, Gao JL, Shen W, Murphy PM, Oppenheim JJ, Wang JM . A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells . J Exp Med 1999 ; 189 : 395 - 402
Uhlar CM, Whitehead AS . Serum amyloid A, the major vertebrate acute-phase reactant . Eur J Biochem 1999 ; 265 : 501 - 523
van der Bend RL, Brunner J, Jalink K, van Corven EJ, Moolenaar WH, van Blitterswijk WJ . Identification of a putative membrane receptor for the bioactive phospholipid, lysophosphatidic acid . EMBO J 1992 ; 11 : 2495 - 2501
Xie Y, Gibbs TC, Mukhin YV, Meier KE . Role for 18:1 lysophosphatidic acid as an autocrine mediator in prostate cancer cells . J Biol Chem 2002 ; 277 : 32516 - 32526
Xu Y, Fang XJ, Casey G, Mills GB . Lysophospholipids activate ovarian and breast cancer cells . Biochem J 1995 ; 309 : 933 - 940
Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O . LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells . J Exp Med 2000 ; 192 : 1069 - 1074
Acknowledgements
This study was supported by research funds from Dong-A University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Shim, J., Jo, S., Kim, S. et al. Lysophosphatidylglycerol inhibits formyl peptide receptor like-1-stimulated chemotactic migration and IL-1β production from human phagocytes. Exp Mol Med 41, 584–591 (2009). https://doi.org/10.3858/emm.2009.41.8.064
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2009.41.8.064
Keywords
This article is cited by
-
Changes of Facial Lipidomics by Intense Pulsed Light Treatment Based on LC-MS
Aesthetic Plastic Surgery (2024)
-
Lipidomic analysis of brain and hippocampus from mice fed with high-fat diet and treated with fecal microbiota transplantation
Nutrition & Metabolism (2023)
-
fMLP-dependent activation of Akt and ERK1/2 through ROS/Rho A pathways is mediated through restricted activation of the FPRL1 (FPR2) receptor
Inflammation Research (2018)
-
Quantitative profiling of glycerophospholipids during mouse and human macrophage differentiation using targeted mass spectrometry
Scientific Reports (2017)