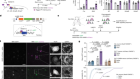
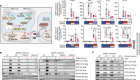
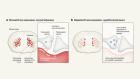
In the last decade, biologists have had remarkable success extracting single cells from tissues and analyzing the genetic material in these individual cells. But chemist Joshua Edel and his collaborators wanted to go one step further. They wanted to extract the mini-structures within cells known as organelles without also extracting cellular fluid or destroying a cell in the process. In a study published this week, they have shown that they can do just this.
The study1, published in Nature Nanotechnology on 3 December, describes a technique developed by Edel and his colleagues at Imperial College London that can extract a single mitochondrion—the power-producing organelle within each cell—from a mouse neuron in a dish using what they call ‘nanotweezers,’ as shown in this video.
They propose that the technique could be used to better understand the underlying causes of neurodegenerative diseases such as Parkinson’s that are associated with malfunctions in mitochondria.
The method described in the study is an “engineering feat,” says Nathan Swami, a bioengineer at the University of Virginia.
These ‘tweezers’ are not quite tools you find in a cosmetics kit. Instead, the two electrodes have been engineered onto the split ends of a thin, glass needle. The electrodes act like tweezers by attracting and isolating parts of a cell to an electric field created between the electrodes, which are roughly 10-20 nanometers apart. As a comparison, a single human hair is roughly 75,000 nanometers in diameter.
Nanoscale tweezers for single-cell biopsies
In previous work done by scientists at the University of California, Irvine, a similar set of ‘nanotweezers’ was used to extract messenger RNA cells from a single cell’s cytoplasm. But no other group seems to have managed to extract single messenger RNA molecules in a way that Ivanov’s and Edel’s groups have demonstrated. The Imperial College group did so in human pulmonary artery cells in a dish.
“The smaller the molecule, the harder it is to trap and extract,” says Alex Ivanov, one of the coauthors of the new paper.
Nano steps
The field of single-cell analysis has taken off in recent years because scientists have come to understand that what determines whether a cancer treatment will work, for instance, or whether an antibiotic will work, comes down to a few cells. “A small subpopulation of cells can play a large role in disease,” Swami says. One potential challenge, according to Orane Guillaume-Gentil, a biologist at the Swiss Federal Institute of Technology in Zurich, is that the technique described in the paper will likely only make it possible to take out a handful of organelles or even DNA or RNA molecules at a given time. The process described in the new paper is labor-intensive, and difficult to scale, says Guillaume-Gentil, who is also working on single-cell extraction techniques.
According to Edel, it takes a day to do about 20-30 extractions, and it is not yet an automated process. “With automation we expect to be able to bring this up to hundreds of biopsies per day,” Edel says.
Despite the time-intensive nature of the process, it could still be useful, say its creators. “Cells contain many mitochondria but it is not well understood whether, and to what extent, individual mitochondria are different from one another,” says Michael Devine, a neurologist and co-author on the Nature Nanotechnology paper. “We can now biopsy individual mitochondria from different locations and directly compare them,” he says, adding that the technique could also be used to reposition mitochondria within cells and then see the impact on neighboring cellular structures.
For now, the authors of the study see their technique as more of an exploratory tool. “It’s becoming increasingly important to understand what is being expressed in the cell and how that changes over time,” Ivanov says. “Our method is a straightforward way to do that.”