It is the world’s thinnest gold leaf: a gossamer sheet of gold just one atom thick. Researchers have synthesized1 the long-sought material, known as goldene, which is expected to capture light in ways that could be useful in applications such as sensing and catalysis.
Goldene is a gilded cousin of graphene, the iconic atom-thin material made of carbon that was discovered in 2004. Since then, scientists have identified hundreds more of these 2D materials. But it has been particularly difficult to produce 2D sheets of metals, because their atoms have always tended to cluster together to make nanoparticles instead.
Researchers have previously reported single-atom-thick layers of tin2 and lead3 stuck to various substances, and they have produced gold sheets sandwiched between other materials. But “we submit that goldene is the first free-standing 2D metal, to the best of our knowledge”, says materials scientist Lars Hultman at Linköping University in Sweden, who is part of the team behind the new research. Crucially, the simple chemical method used to make goldene should be amenable to larger-scale production, the researchers reported in Nature Synthesis on 16 April1.
“I’m very excited about it,” says Stephanie Reich, a solid-state physicist and materials scientist at the Free University of Berlin, who was not involved in the work. “People have been thinking for quite some time how to take traditional metals and make them into really well-ordered 2D monolayers.”
In 2022, researchers at New York University Abu Dhabi (NYUAD) said that they had produced goldene, but the Linköping team contends that the prior material probably contained multiple atomic layers, on the basis of the electron microscopy images and other data that were published in ACS Applied Materials and Interfaces4. Reich agrees that the 2022 study failed to prove that the material was single-layer goldene.
However, Ramesh Jagannathan, who led the NYUAD study, stands by the work and disputes the Linköping team’s assertions about it. “We did extensive characterization using atomic force microscopy,” he says, referring to a technique that images surfaces by scanning them with a cantilever. The NYUAD researchers isolated their material on top of an atomically-flat piece of sapphire; they also demonstrated that it is a semiconductor, as expected for monolayer gold.
Golden age
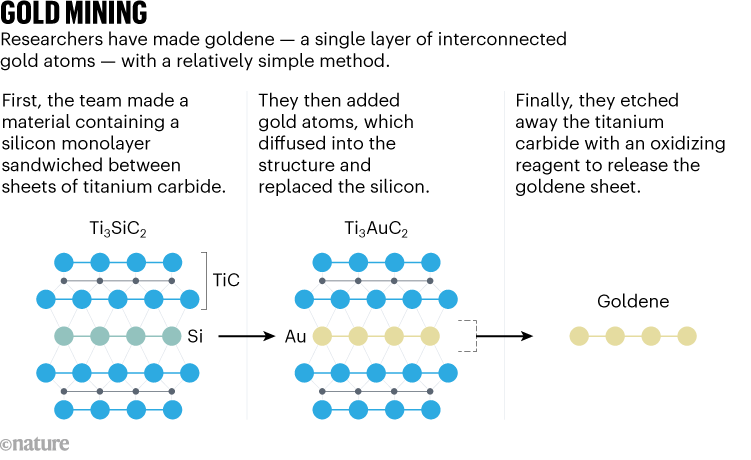
To prepare goldene, the Linköping researchers started with a material containing atomic monolayers of silicon sandwiched between titanium carbide. When they added gold on top of this sandwich, it diffused into the structure and exchanged places with the silicon to create a trapped atom-thick layer of gold (see ‘Gold mining’). They then etched away the titanium carbide to release free-standing goldene sheets that were up to 100 nanometres wide, and roughly 400 times as thin as the thinnest commercial gold leaf, Hultman estimates.
Source: Adapted from Ref. 1.
That etching process used a solution of alkaline potassium ferricyanide known as Murukami’s reagent. “What’s so fascinating is that it’s a 100-year-old recipe used by Japanese smiths to decorate ironwork,” Hultman says. The researchers also added surfactant molecules — compounds that formed a protective barrier between goldene and the surrounding liquid — to stop the sheets from sticking together.
The Linköping team suggests that goldene might be useful in applications in which gold nanoparticles already show promise. Light can generate waves in the sea of electrons at a gold nanoparticle’s surface, which can channel and concentrate that energy. This strong response to light has been harnessed in gold photocatalysts to split water to produce hydrogen, for instance. Goldene could open up opportunities in areas such as this, Hultman says, but its properties need to be investigated in more detail first.
“I think the research is really interesting,” says Graham Hutchings, a chemist at the University of Cardiff, UK, who develops gold catalysts. But he worries that any residual traces of iron from Murukami’s reagent might hamper the development of goldene as a catalyst. “I would think that potential contamination with iron is going to cause a few problems in applications,” he says.
For now, the Linköping researchers are seeking better ways to sieve goldene from the solution used to make it, and to grow larger flakes of the material. They are also exploring whether their method can be used to make monolayers of other catalytic metals, including iridium, platinum and palladium.


 Weird new electron behaviour in stacked graphene thrills physicists
Weird new electron behaviour in stacked graphene thrills physicists
 Strange topological materials are popping up everywhere physicists look
Strange topological materials are popping up everywhere physicists look
 How ‘magic angle’ graphene is stirring up physics
How ‘magic angle’ graphene is stirring up physics