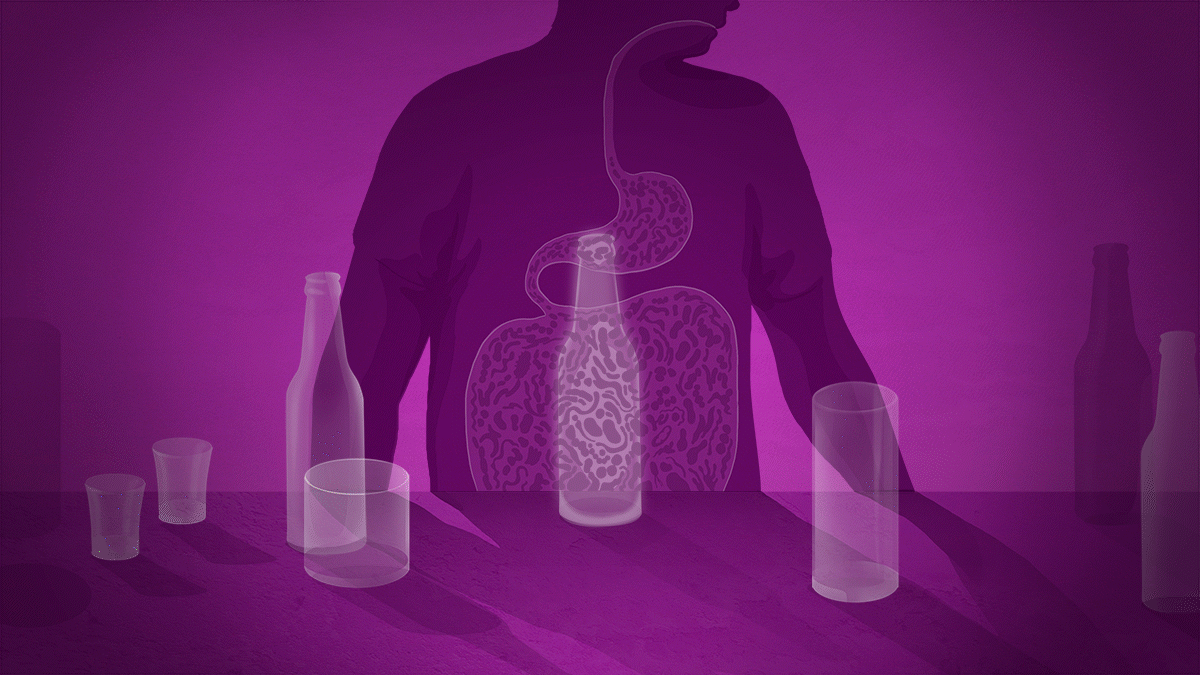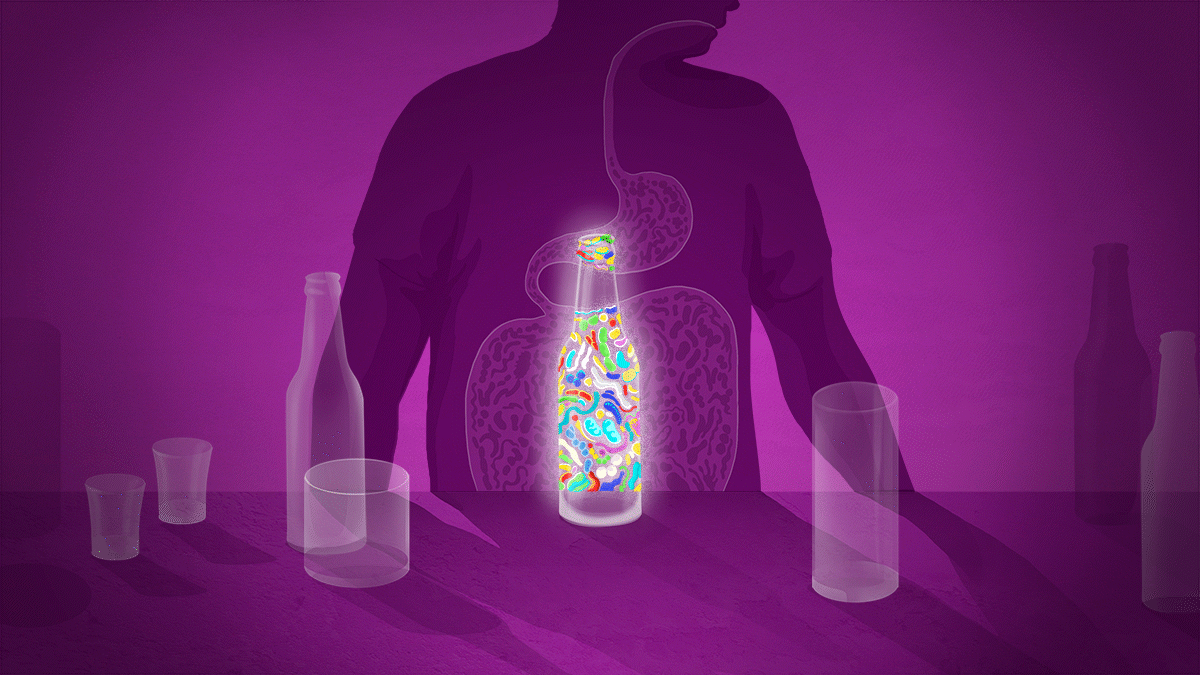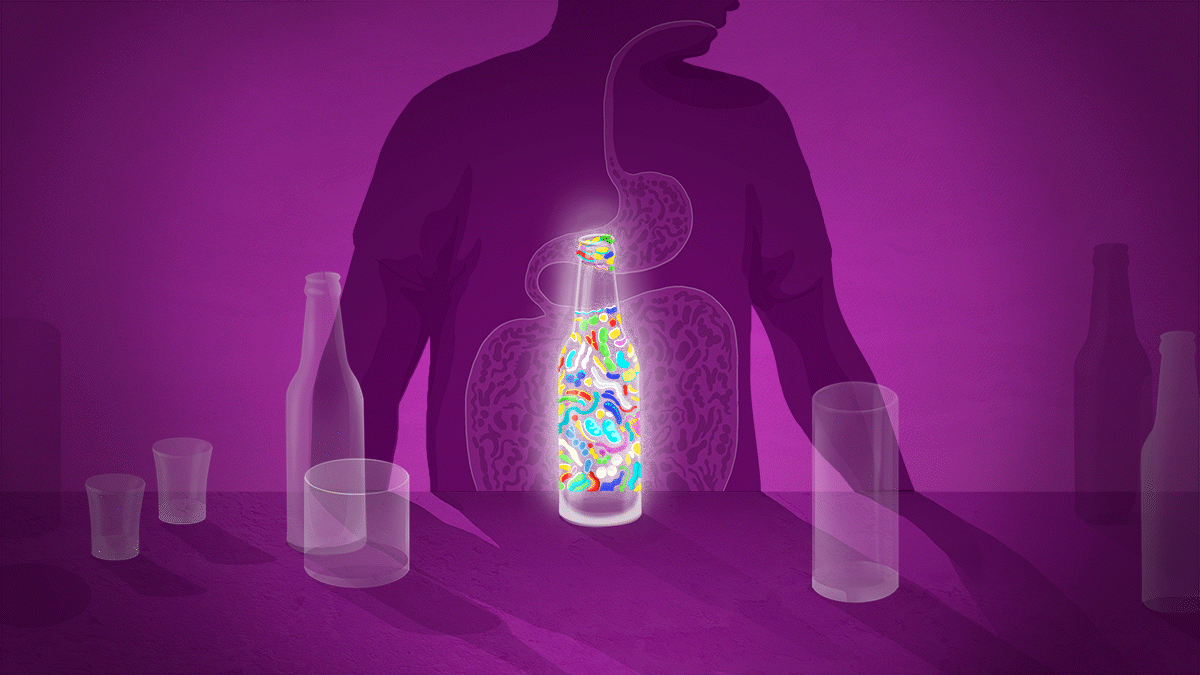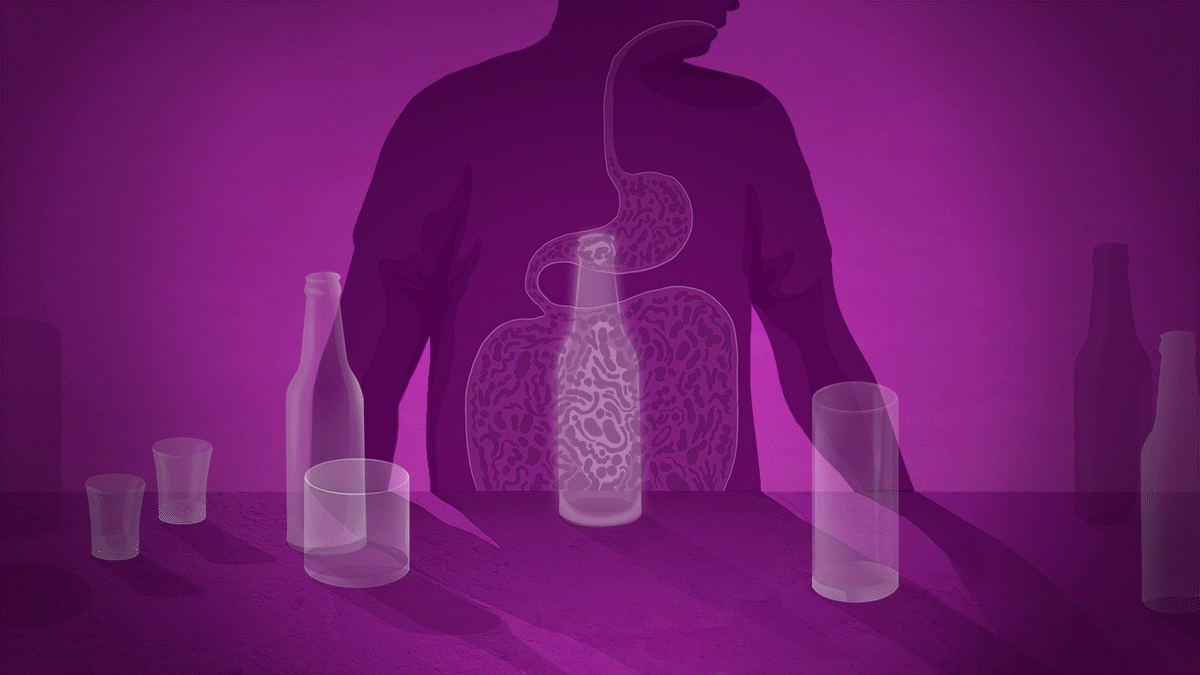
Illustration: Sam Falconer
Andrew Day, a molecular microbiologist at Tufts University in Medford, Massachusetts, is four years sober. His journey to this point inspires his work, which he hopes might help others who are struggling with alcohol.
Nature Outlook: The human microbiome
There are many risk factors associated with alcohol-use disorder (AUD), including mental-health conditions and genetics. But Day is eyeing a more unusual contributor: the gut.
Over the past decade, research has begun to highlight a link between the gastrointestinal microbiome — the microorganisms that live inside our digestive tract — and addiction. Researchers including Day suggest that an imbalance in the intestinal microbiota, known as dysbiosis, might cause the gut to send signals to the brain that promote addiction behaviours. If correct, the gut could become a treatment target for people with AUD. “I could find something that might make it easier for people who might not be as fortunate to maintain sobriety,” says Day, who is studying the theory that high levels of the fungus Candida albicans in the gut contributes to increased alcohol consumption in mice as part of his PhD.
This is a sharp departure from conventional medical approaches to treating addiction. Most drugs for AUD and substance-use disorder (SUD) focus on brain chemistry. Many of them are not very effective. Medications for AUD approved by the US Food and Drug Administration (FDA) include naltrexone and acamprosate. In addition, the European Medicines Agency (EMA) has approved nalmefene. Acamprosate modulates brain receptors such as those that bind γ-aminobutyric acid (GABA), an inhibitory neurotransmitter thought to have a role in withdrawal, craving and impulsive behaviour. Nalmefene and naltrexone modulate opioid receptors, nalmefene reduces alcohol cravings, and naltrexone blocks euphoric sensations associated with alcohol.
According to the US Substance Abuse and Mental Health Services Administration, only 42% of people who receive treatment for any kind of SUD complete that treatment1. Between 40% and 60% of people with an SUD will relapse, and it can take years — sometimes decades — of see-sawing between abstinence and relapse before someone achieves sustained remission. Clearly, there is room for improvement. “We’ve missed the target for 50 years,” says Benjamin Boutrel, a neurobiologist at Lausanne University Hospital in Switzerland. “Mostly because it’s not only a matter of the brain — it’s possibly a matter of the guts, too.”
The gut–brain axis
It is now well known that there is complex communication between the gut and the brain, through the vagus nerve as well as through the endocrine and immune systems. This gut–brain signalling has been suggested to influence addiction-related behaviours in two main ways.

Andrew Day hopes his research will help others who have alcohol-use disorder.Credit: Dr. Carol Kumamoto
The first involves a condition known as leaky gut. Stress, poor diet, food allergies, chemotherapy and other medication, conditions such as inflammatory bowel disease and — perhaps crucially — overuse of alcohol can damage the layer of epithelial cells that line the intestines. This can make the intestinal wall permeable to food particles and bacteria, which can then sneak into the circulatory system.
When this happens, immune cells secrete inflammatory mediators such as cytokines. These proteins can then reach the brain, either through the vagus nerve or by crossing weak areas in the blood–brain barrier, a layer of cells meant to protect the brain from damage.
The subsequent inflammation can affect the brain in several ways that could promote addiction. Cytokines deplete tryptophan, which can lead to reduced production of the mood-regulating hormone serotonin. The brain’s amygdala might sense a threat in the body and increase its activity in response to inflammation. The ventral striatum — the area of the brain related to reward anticipation — might also be ignited. The anterior cingulate cortex — the part of the brain involved in inhibitory control and compulsive behaviour — can also activate during inflammation.
Second, the molecules that gut microbes produce could influence addiction. Some of these are important for brain functioning. The gut bacteria Lactobacillus, for example, can produce GABA; Enterococcus can produce serotonin; and Bacillus can make dopamine. Short-chain fatty acids (SCFAs) released when dietary fibre is fermented by bacteria in the gut also have neuroactive properties.
Gut dysbiosis, and its subsequent impact on GABA, serotonin, dopamine and tryptophan, could, therefore, make a person more susceptible to addiction and mean that they experience more severe withdrawal symptoms than would someone with a healthy gut microbiome.
“The gut microbiome is really important for some organs, including the brain,” says Drew Kiraly, a psychiatrist and physician at Wake Forest University in Winston-Salem, North Carolina. Kiraly has observed associations between dysbiosis and addictive behaviour to stimulants and opioids in rats. He has used antibiotics to deplete rats’ beneficial gut microbes, resulting in “aberrant responses to drugs”. The animals had increased intake of cocaine and fentanyl, he says. “And after withdrawal, they relapse and have higher fentanyl-seeking behaviour.”
Addictive personality
Even before first contact with alcohol or drugs, pre-existing dysbiosis could make someone more vulnerable to addiction, Boutrel says. The imbalance could give rise to traits such as impulsivity, boredom, susceptibility to stress or anxiety, and sensation seeking. “Those who get thrilled with poker playing, with pathological sex, they all need something,” Boutrel says. “There is a vulnerability there that, once that first contact is made, will trigger repetition — and finally, addiction.”

Sophie Leclercq is one of few researchers able to study theories about the gut microbiome in people with alcohol-use disorder.Credit: Sophie Leclercq
In 2018, Boutrel and his colleagues put a group of 59 rats through a number of tests designed to assess their vulnerability to AUD2. First, the rodents were trained to self-administer alcohol by pressing a lever. The researchers then tried to gauge the rats’ self-control by introducing a delay to the reward delivery. Some rats pressed the button once, realized that they had to wait, and went about their business. But some would continue pressing over and over, attempting to make the alcohol arrive more quickly — an indication of addiction.
The final test, which Boutrel thinks is most telling, introduced a deterrent — an uncomfortable foot shock every time the animals took the alcohol. For most of the rats, this discouragement was sufficient and they stopped pressing the lever. However, a sizable minority “just didn’t care”, Boutrel says. “They could not stop pressing the lever and accessing the reward, even when they got a punishment.” In total, about 30% of the rats demonstrated vulnerability to AUD.
Having identified a group of vulnerable rats, Boutrel and his colleagues removed alcohol from the rats environment for three months, and then compared the brains and gut microbiomes of the vulnerable rats with those of rats that had proven more resistant to AUD. The team found that the vulnerable rats had more efficient dopamine 1 receptors (which trigger increased reward-seeking and motivation) and less efficient dopamine 2 receptors (which cause impulsivity, and an increased need for immediate rewards and drug administration). They also found differences in the bacterial content of the vulnerable-rat guts — most notably, changes in Lachnospiraceae, Syntrophococcus and other bacteria associated with reductions in dopamine 2 receptors. This, the researchers suggest, is an indication that gut microbiota could affect brain circuits associated with addiction.
Alcohol and other drugs
Sophie Leclercq, a biomedical scientist at the Catholic University of Louvain in Brussels, was an early advocate of the theory about an AUD gut–brain origin, and one of the first to test it in people3. Her aim was to find out whether intestinal permeability was related to character traits that might make people more susceptible to alcohol dependence.
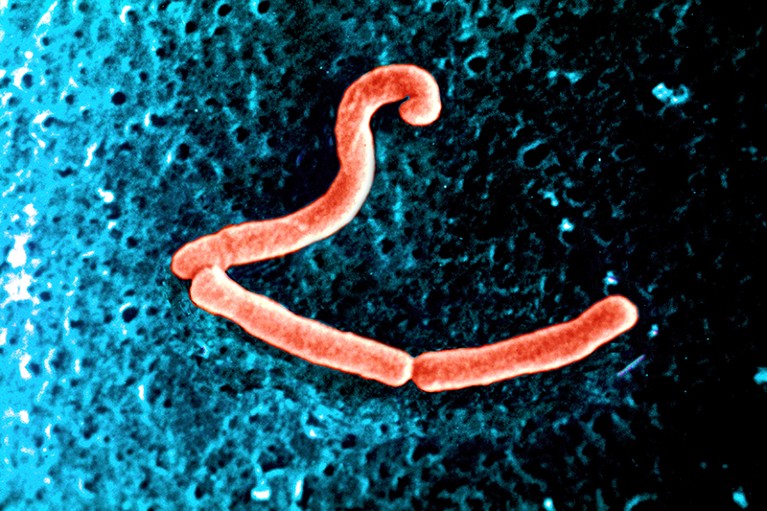
Lactobacillus gut bacteria can produce the inhibitory neurotransmitter GABA.Credit: BSIP/UIG Via Getty Images
Leclercq and her colleagues tested the intestinal permeability of 60 people with AUD two days after they began withdrawal. The researchers found that 26 (43%) had high intestinal permeability. At the beginning of the study, everyone with AUD had higher scores of depression, anxiety and craving than did people in the control group. At the end of three weeks of abstinence, the scores of people with low intestinal permeability returned to levels equal to those of the control group. People with high intestinal permeability, however, still scored highly in tests of depression, anxiety and craving, which are directly related to the urge to drink and have a major role in whether people can abstain after detoxification.
“We wanted to see if there was some connection between the gut microbiota and the psychology of AUD, and, indeed, we found that there is a very strong association between dysbiosis, the alteration of the gut microbiota composition, and symptoms like depression, anxiety or grief,” Leclercq says.
Although much of this research is related to people with AUD, Kiraly says that they’ve seen similar results in people who misuse opioids, and cocaine and other stimulants. “Depletion [of microbiota] seems to dysregulate these networks that underlie behavioural changes,” he says.
In 2023, Kiraly and his colleagues looked at whether rats’ microbiomes affected the animal’s drug-seeking behaviours4. In one experiment, rats were given either clean water or water containing the antibiotics neomycin, vancomycin, bacitracin and pimaricin, all of which would deplete their gut microbiota. They were then let into a chamber in which they could push a lever that lit up and provided 0.8 milligrams of cocaine. Later, researchers altered how the lever behaved — now it would light up when pushed, but would have to be pushed more times for the rats to receive cocaine. Researchers found that the rats with depleted gut microbiota were much more likely to press the lever repeatedly to receive cocaine than were the rats given only water.
In a second experiment, both groups of rats were able to self-administer cocaine for two weeks, then detoxed for 21 days. When the rats returned to the cages in which cocaine was available, those receiving antibiotics headed to the lever that originally dosed cocaine twice as quickly as the other rats did. These rats also pressed the lever much more frequently than the control rats did.
“We wanted to study a model of relapse and we saw that microbiome-depleted animals work harder for a drug-related cue than the others did,” Kiraly said. “Lots of people use drugs and not all get to the stage of problematic use. It could be that your microbiome predisposes you.”
Treatment questions
There is still a lot of research that needs to be done before any microbiome-targeted treatment could be offered to people with AUD or another SUD. Researchers don’t yet know, for example, which microbiota are most important, and which gut–brain pathways they need to target. “People have asked me, ‘Can someone just eat yogurt and cure their addiction?’” Kiraly says. “It’s going to be much, much more complicated than that.”
Kiraly would like to see whether probiotics or other treatments could have potential for people with early problematic use but who have not yet progressed to AUD. For instance, some rats in Kiraly’s study were administered SCFAs alongside their antibiotics. Compared with rats that received only antibiotics, those also given SCFAs seemed to retain more Firmicutes and less Proteobacteria (many of which are pathogenic). Strikingly, when the post-detox rats were given the chance to consume cocaine again, those who had received SCFAs behaved like rats with normal gut flora.
Leclercq thinks that 30–40% of cases of AUD might have a gut-related component that could be targeted for treatment. A key challenge is determining exactly which components to target — it is as yet unclear what constitutes a ‘good’ microbiome. Day’s analysis suggests that bacteria such as Lactobacillus, were in abundance in people with AUD, whereas Akkermansia and some others were low.
There is also uncertainty regarding what would be the most effective and easiest part to target of the chain of communication between the gut and brain. Areas such as the nervous system, blood stream or the system surrounding the gut are all candidates.
It is also tricky to find people with AUD who are willing to not only abstain from drinking, but also take part in research, including providing samples of their gut microbiome. Leclercq is one of few researchers able to work with people, instead of rats, because she is affiliated with a hospital with a detoxification clinic. But even she can find it difficult to get enough volunteers for studies. In work assessing the effects of a prebiotic on people with AUD, the number of people with dysbiosis was around half that of those who had healthy guts, making comparisons between the two difficult. Leclercq’s analysis of this aspect of the study is yet to be published.
Despite these issues, Leclercq is moving forward with her research, and is now looking at nutrition as a way to improve the gut microbiome. She is starting a study on polyunsaturated fatty acids — such as those abundant in rapeseed and maize (corn) oils, walnuts, tofu and fatty fish, including salmon and mackerel — and hopes to have results in about two years. She’s also working to correlate which metabolites from food are related to depression, anxiety and craving, and trying to find funding for a study to test these particular nutritional compounds in people.
“Pharmaceutical companies have tried to target GABA, dopamine and serotonin, and these treatments aren’t very efficient because the relapse rate is very high in this disease,” she says. For people with AUD whose guts are contributing to their condition, nutritional interventions, probiotics and prebiotics could eventually improve the odds of success.

 COVID lockdowns altered babies’ microbiomes
COVID lockdowns altered babies’ microbiomes