- NEWS AND VIEWS
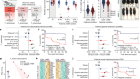
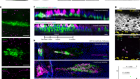
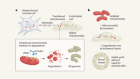
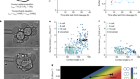
Developing cells remember where they came from, thanks to keratin filaments
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 585, 352-353 (2020)
doi: https://doi.org/10.1038/d41586-020-02443-w
References
Lim, H. Y. G. et al. Nature 585, 404–409 (2020).
Johnson, M. H. & Ziomek, C. A. Cell 24, 71–80 (1981).
Zenker, J. et al. Cell 173, 776–791 (2018).
Jackson, B. W. et al. Differentiation 17, 161–179 (1980).
Serres, M. P. et al. Dev. Cell 52, 210–222 (2020).
Duarte, S. et al. Nature Commun. 10, 4200 (2019).
Nishioka, N. et al. Dev. Cell 16, 398–410 (2009).
Yamada, S., Wirtz, D. & Coulombe, P. A. J. Struct. Biol. 143, 45–55 (2003).
Knoblich, J. A. Nature Rev. Mol. Cell Biol. 11, 849–860 (2010).
Skamagki, M., Wicher, K. B., Jedrusik, A., Ganguly, S. & Zernicka-Goetz, M. Cell Rep. 3, 442–457 (2013).
Maître, J.-L. et al. Nature 536, 344–348 (2016).
Otzen, D. & Riek, R. Cold Spring Harb. Perspect. Biol. 11, a033860 (2019).

 Read the paper: Keratins are asymmetrically inherited fate determinants in the mammalian embryo
Read the paper: Keratins are asymmetrically inherited fate determinants in the mammalian embryo
 Tissue ‘melting’ sculpts embryos
Tissue ‘melting’ sculpts embryos
 Mechanics drives cell differentiation
Mechanics drives cell differentiation