- NEWS AND VIEWS
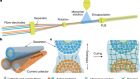
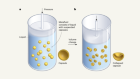
Droplet motion electrically controlled
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 572, 445-446 (2019)
doi: https://doi.org/10.1038/d41586-019-02451-5
References
Yao, X., Song, Y. & Jiang, L. Adv. Mater. 23, 719–734 (2011).
Cho, S. K., Moon, H. & Kim, C.-J. J. Microelectromech. Syst. 12, 70–80 (2003).
Pollack, M. G., Fair, R. B. & Shenderov, A. D. Appl. Phys. Lett. 77, 1725–1726 (2000).
Berge, B. & Peseux, J. Eur. Phys. J. E 3, 159–163 (2000).
Hayes, R. A. & Feenstra, B. J. Nature 425, 383–385 (2003).
Krupenkin, T. & Taylor, J. A. Nature Commun. 2, 448 (2011).
Mugele, F. & Heikenfeld, J. Electrowetting: Fundamental Principles and Practical Applications (Wiley, 2018).
Li, J., Ha, N. S., Liu, T., van Dam, R. M. & Kim, C.-J. Nature 572, 507–510 (2019).
De Gennes, P. G. Rev. Mod. Phys. 57, 827–863 (1985).

 Read the paper: Ionic-surfactant-mediated electro-dewetting for digital microfluidics
Read the paper: Ionic-surfactant-mediated electro-dewetting for digital microfluidics
 Water flows out of touch
Water flows out of touch
 Droplets leap into action
Droplets leap into action